Titanate luminescent material and preparation method thereof
A technology of luminescent materials and titanates, which is applied in the direction of luminescent materials, alkaline earth metal titanates, titanates, etc., can solve the problems of increased non-radiative transition probability, ion vacancies and oxygen vacancies, and reduced ion luminescence efficiency, etc., to achieve The effect of improving internal quantum efficiency, good luminous performance, and reducing the probability of non-radiative transition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
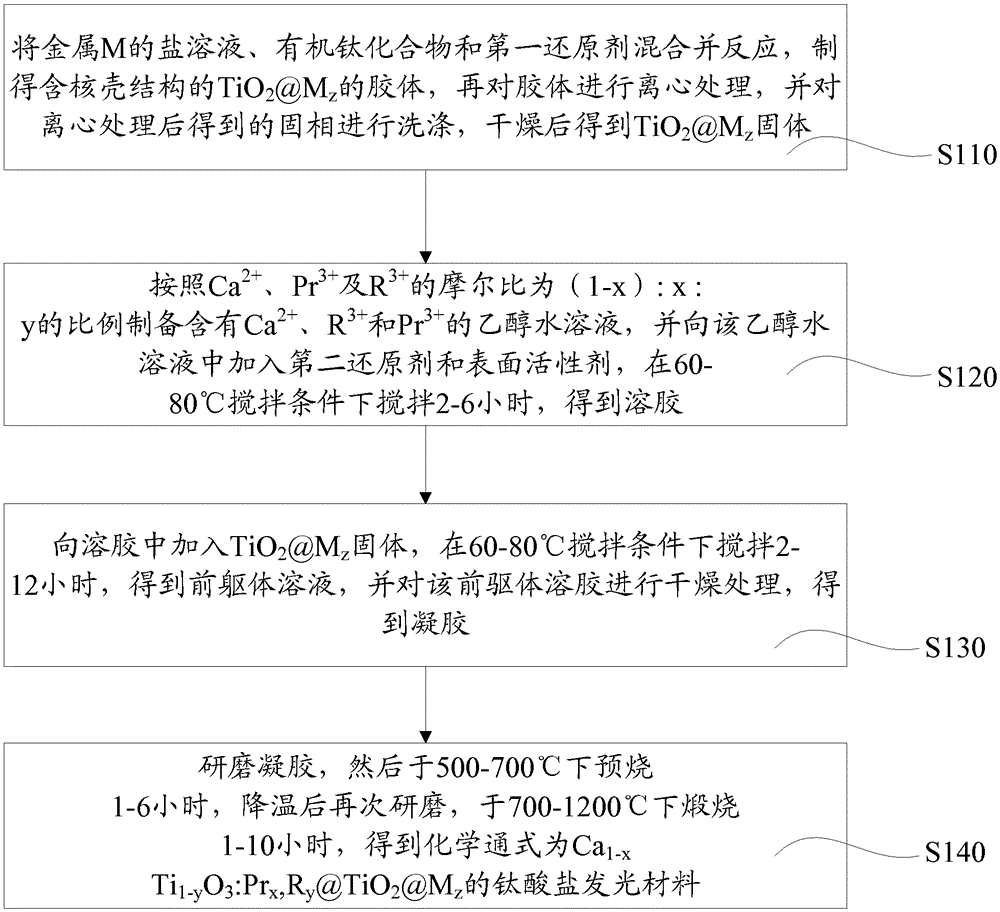
[0029] In addition, this embodiment also provides a method for preparing a titanate luminescent material, such as figure 1 shown, including the following steps:
[0030] Step S110, mixing and reacting the salt solution of the metal M, the organic titanium compound and the first reducing agent to prepare TiO containing a core-shell structure 2 @M z The colloid, and then the colloid is centrifuged, and the solid phase obtained after the centrifugation is washed, and dried to obtain TiO 2 @M z solid. Wherein, the salt solution of the metal M is mixed with the organotitanium compound according to the molar amount that the molar ratio of M to titanium is z, 0-2 , M is at least one of Ag, Au, Pt, Pd and Cu, @ is cladding, M constitutes the core of the core-shell structure, TiO 2 The cladding M constitutes the shell layer of the core-shell structure.
[0031] In this embodiment, the salt solution of metal M has a concentration of 5×10 -5 mol / L-5×10 -3 mol / L containing HAuCl 4...
Embodiment 1
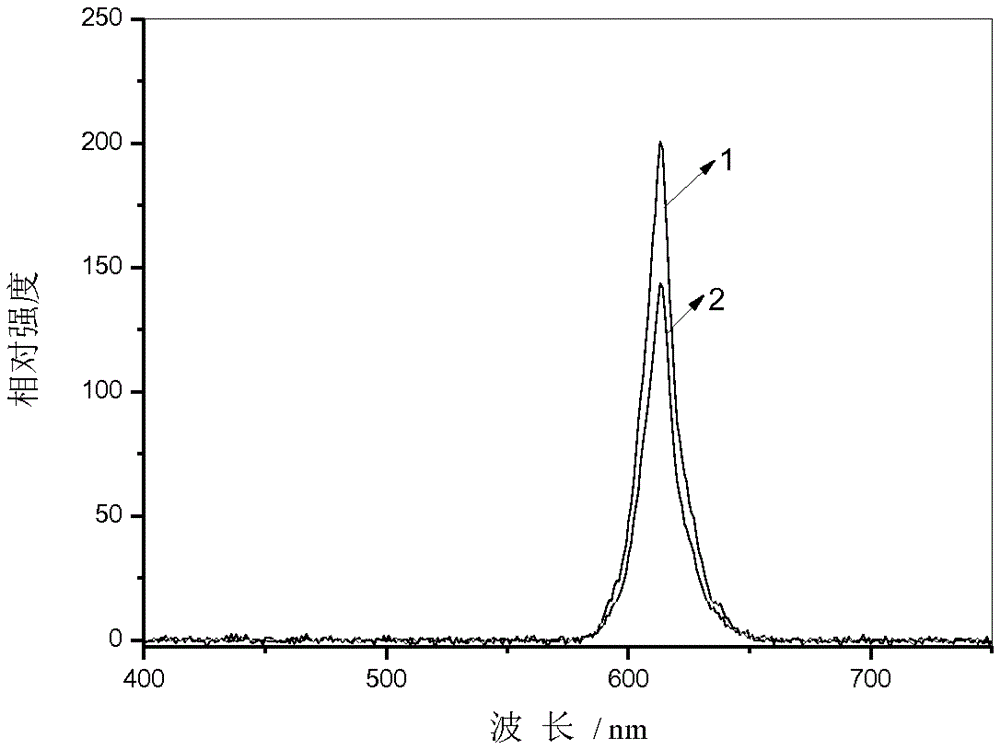
[0041] Preparation of Ca by sol-gel method 0.999 Ti 0.98 o 3 :Pr 0.001 ,Al 0.02 @TiO 2 @ :
[0042] TiO 2 @ Preparation: Weigh 10.3mg of chloroauric acid (AuCl 3 ·HCl·4H 2 O) Dissolve in deionized water to prepare 20mL 5×10 -3 mol / L chloroauric acid solution; pipette 5mL 4.3mol / L triethanolamine titanium isopropoxide, dilute to 1mol / L with isopropanol; pipette 10mL 5×10 -3 mol / L chloroauric acid solution and 5mL 1mol / L isopropanol solution of triethanolamine titanium isopropoxide, stir evenly; then add 15mL dimethylformamide to the mixed solution, stir at room temperature for 15 minutes, then use The condensing reflux device is heated and stirred at 140 ° C, the reaction liquid is colorless to light brown, and then to dark brown, stop heating, cool to room temperature, and obtain TiO 2 @ colloid; then centrifuged TiO 2 @ The solid phase was collected by the colloid, and the collected solid phase was washed with ethanol, and dried to obtain TiO 2 @ solid. ...
Embodiment 2
[0045] Preparation of Ca by sol-gel method 0.998 Ti 0.9 o 3 :Pr 0.002 ,Al 0.1 @TiO 2 @
[0046] TiO 2 @ Preparation: weigh 3.4mg of silver nitrate (AgNO 3 ) was dissolved in deionized water to prepare 20 mL of 1×10 -3 mol / L silver nitrate solution; pipette 10m L of 4.3mol / L triethanolamine titanium isopropoxide, dilute to 0.22mol / L with isopropanol; pipette 2mL 1×10 -3 mol / L silver nitrate solution and 18mL1mol / L isopropanol solution of triethanolamine titanium isopropoxide, stir evenly; then add 10mL dimethylformamide to the mixed solution, stir at room temperature for 15 minutes, then use condensing reflux The device is heated and stirred at 140°C, and the reaction liquid changes from colorless to light brown, and then to dark brown, stop heating, cool to room temperature, and obtain TiO 2 @ colloid; then centrifuged TiO 2 @ The solid phase was collected by the colloid, and the collected solid phase was washed with ethanol, and dried to obtain TiO 2 @ so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com