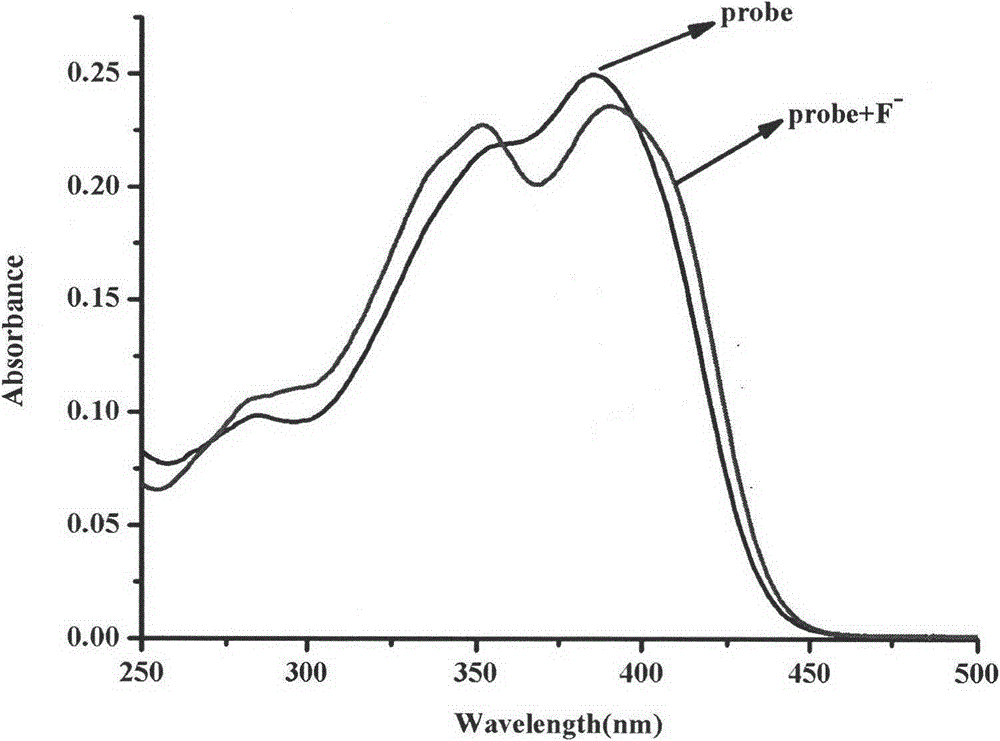
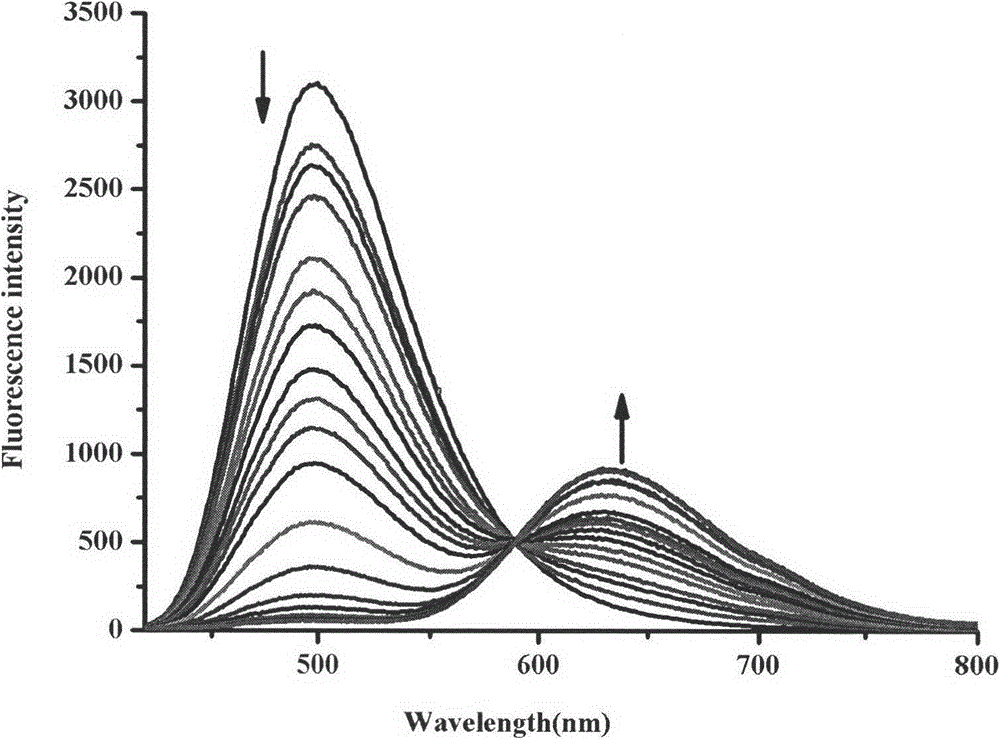
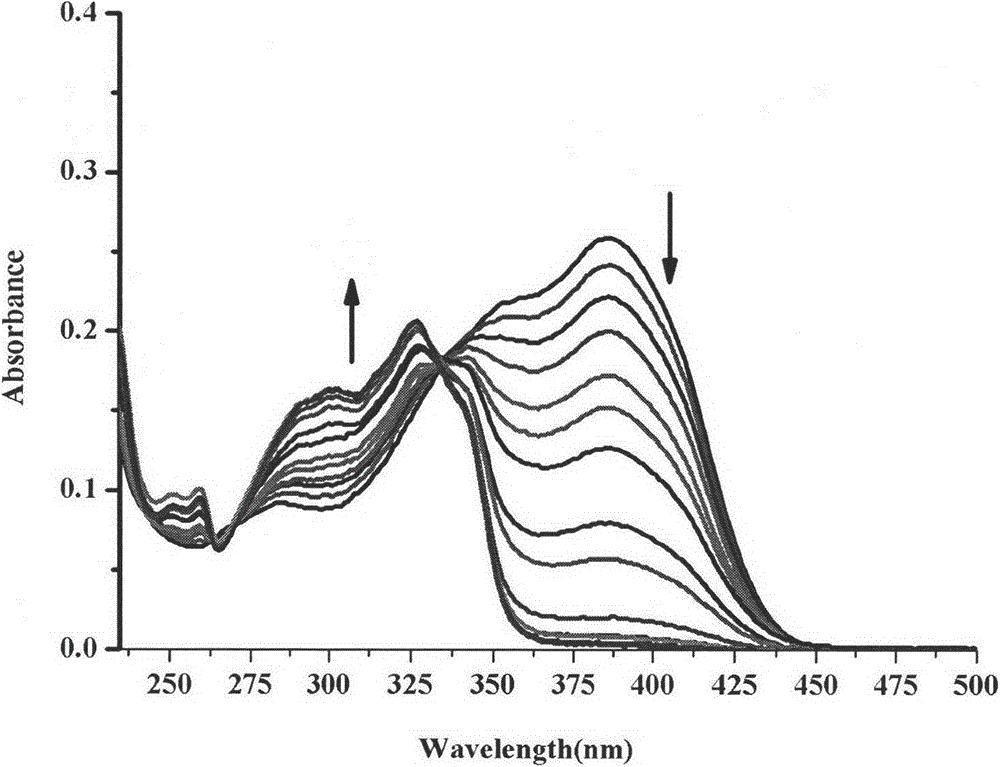
Synthesis method and application of ratiometric fluorescent molecular probe for simultaneously detecting fluorine ion and sulfite radical
A fluorescent molecular probe and sulfite technology, applied in the field of chemical analysis and detection, to achieve the effects of high synthesis yield, easy availability of raw materials, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of 2-(4-methyl-2-hydroxyphenyl)-benzothiazole
[0043] According to the method reported in the literature, 2-(4-methyl-2-hydroxyphenyl)-benzothiazole was synthesized. Weigh 8.2g (66mmol) o-aminothiophenol and 10.0g (66mmol) 4-methylsalicylic acid in 70ml of toluene, heat to 50-55°C under the protection of nitrogen, and stir for about 2.5h. Then, the temperature was lowered to about 35°C, and 6.2ml of phosphorus trichloride was slowly added dropwise to the above system. After the dropwise addition of the phosphorus trichloride, the mixed solution became white and viscous. Continue to heat to 85°C and stir for 5 hours. , The system becomes a clear solution. After the reaction is complete, spin off the solvent and separate by column chromatography (dichloromethane: petroleum ether = 1:10) to obtain 6.4 g of 2-(4-methyl-2-hydroxyphenyl)-benzothiazole with a yield It is 40.3%.
Embodiment 2
[0044] Example 2: Preparation of 2-(4-methyl-2-acetoxyphenyl)benzothiazole (Compound 5)
[0045] Weigh 5.0g (20.7mmol) of 2-(4-methyl-2-hydroxyphenyl)-benzothiazole dissolved in 60ml of dichloromethane, add 2.5g (24.9mmol) of triethylamine, stir for 5min, 1.95g (24.9mmol) of acetyl chloride was added to the system, stirred at room temperature for 2h, monitored by TLC spot plate, and found that the raw material spots basically disappeared. The reaction was quenched by adding 40 ml of water, extracted with dichloromethane, washed with saturated brine, dried over anhydrous sodium sulfate, spin-dried the solvent, and separated and purified by column chromatography (ethyl acetate: petroleum ether = 1:16) to obtain 4.7 g of compound 5. The yield was 80%. 1 H NMR(400MHz, CDCl 3 )δ8.23(d, J=8.1Hz, 1H), 8.11(d, J=8.1Hz, 1H), 7.94(d, J=7.5Hz, 1H), 7.53(t, J=7.7Hz, 1H) , 7.46-7.39 (m, 1H), 7.24 (d, J=8.1, 1H), 7.09 (s, 1H), 2.51 (s, 3H), 2.46 (s, 3H).
Embodiment 3
[0046] Example 3: Preparation of 2-(4-bromomethyl-2-acetoxyphenyl)benzothiazole (Compound 4)
[0047] Weigh 3.5g (12.4mmol) of compound 5, 0.06g (0.025mmol) of benzoyl peroxide, and 2.4g (13.5mmol) of N-bromosuccinimide dissolved in 50ml of dry carbon tetrachloride solution, and reflux Stir for 24h. After the reaction, the mixture was poured into ice water, extracted with chloroform, dried over anhydrous sodium sulfate, spin-dried the solvent, and separated and purified by column chromatography (ethyl acetate: petroleum ether = 1:14) to obtain 2.3 g of compound 4, with a yield It is 51.3%. 1 H NMR(400MHz, CDCl 3 )δ8.35 (d, J = 8.1 Hz, 1H), 8.12 (d, J = 7.9 Hz, 1H), 7.96 (d, J = 7.4 Hz, 1H), 7.58-7.52 (m, 1H), 7.48- 7.42 (m, 2H), 7.33 (s, 1H), 4.54 (s, 2H), 2.52 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com