Carbonic ester photosensitive reagent as well as preparation method thereof and method for preparing 5'-photosensitive protected nucleoside
A carbonate type and reagent technology, which is applied in the field of carbonate type photosensitizers and their preparation, and the preparation of 5'-photosensitive protected nucleosides, can solve the problems of inconvenient operation, difficult separation, low yield and the like, and can reduce production Cost, reduction of purification process, effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 5'-NPPOC-dT( 5 ) preparation
[0032] 1a. Preparation reaction steps of photosensitive reagent:
[0033]
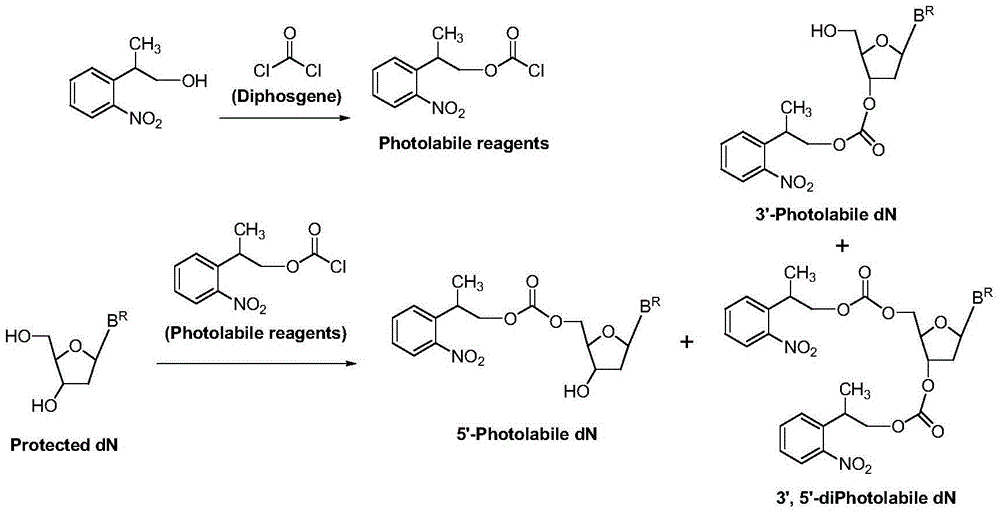
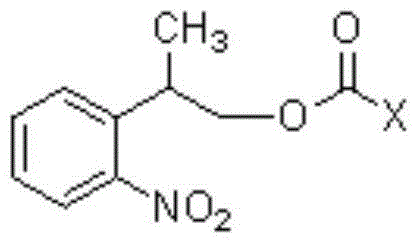
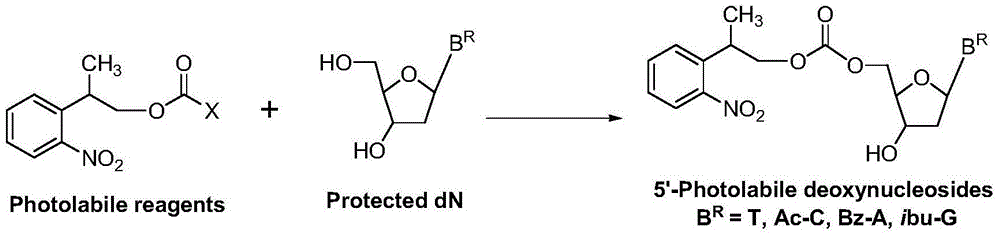
[0034] At room temperature 25°C, in a reaction vessel, 2-o-nitrobenzene-1-propanol 1 [2-(2-nitrophenyl)propan-1-ol, 54.4g, 0.30mol] was dissolved in dichloromethane (544ml), bis(p-nitrophenyl) carbonate was added 2 [Bis(4-nitrophenyl)carbonate, 0.33mol], then added triethylamine (0.303mol); the reaction system was stirred at room temperature, and after 6 hours, HPLC showed that the reaction was complete, and the reaction was stopped. The reaction solution was successively water (2×300ml, indicated by After extraction with 300ml of water, extract once with 300ml of water (extracted twice in total) and saturated aqueous sodium chloride solution (300ml), keep the organic (dichloromethane) layer, dry the dichloromethane layer with anhydrous sodium sulfate, and filter , the filtrate was evaporated to dryness, and dried in vacuo to obtain (2-o-nitrophenyl)-1-propyl...
Embodiment 2
[0042] 5'-NPPOC-N-Ac-dC( 9 ) preparation
[0043] 2a. Preparation reaction steps of photosensitizer:
[0044]
[0045] At room temperature 20°C, in a reaction vessel, 2-o-nitrobenzene-1-propanol 1 [2-(2-nitrophenyl)propan-1-ol, 0.30mol] was dissolved in chloroform (544ml), and bis(pentafluorobenzene)carbonate was added 6 [Bis(pentafluorophenyl)carbonate0.33mol], and then added triethylamine (0.303mol). The reaction system was stirred at room temperature. After 9 hours, HPLC showed that the reaction was complete, and the reaction was stopped. The reaction solution was extracted with water (2×300ml) and saturated aqueous sodium chloride solution (300ml) successively, and the organic (chloroform) layer was retained; After drying with anhydrous sodium sulfate, filter, evaporate to dryness, and vacuum dry to obtain (2-o-nitrophenyl)-1-propyl pentafluorobenzenecarbonate 7 , 95.1g, the HPLC purity was 97%, and the yield was 81%.
[0046] 2b. Select the coupling reaction step:...
Embodiment 3
[0053] 5'-NPPOC-N-Bz-dA( 13 ) preparation
[0054] 3a. Preparation reaction steps of photosensitizer:
[0055]
[0056] At room temperature 25°C, in a reaction vessel, 2-o-nitrobenzene-1-propanol 1 [2-(2-nitrophenyl)propan-1-ol, 0.30mol] was dissolved in ethyl acetate (540ml), and N'N-carbonyldiimidazole was added 10 (1,1'-Carbonyldiimidazole, 0.33 mol), and triethylamine (0.303 mol) was added. The reaction system was stirred at room temperature. After 7 hours, HPLC showed that the reaction was complete, and the reaction was stopped. The reaction solution was extracted with water (2×300ml) and saturated aqueous sodium chloride solution (300ml) successively, and the organic (ethyl acetate) layer was retained; After drying with anhydrous sodium sulfate, filter, evaporate to dryness, and vacuum dry to obtain N-imidazole carbonate (2-o-nitrophenyl)-1-propyl ester 11 , 69.4g, the HPLC purity was 96%, and the yield was 84%.
[0057] 3b. Select the coupling reaction step:
...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap