Cyclic polyphosphoester oligmer, and preparation method and application thereof
A polyphosphate and oligomer technology, applied in the field of flame retardants, can solve the problems of unfavorable mechanical properties of polymer materials, low decomposition temperature of flame retardants, influence on flame retardant effect, etc., and achieve high yield and thermal stability. Good, improve the effect of mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention also provides a method for preparing the cyclic polyphosphate oligomer, which comprises the following steps:
[0031] The compound having the structure of formula (II) is mixed with the compound having the structure of formula (III), and the polymerization reaction is carried out in the presence of a catalyst or acid scavenger to obtain a cyclic polyphosphate oligomer having a structure of formula (I) ,
[0032]
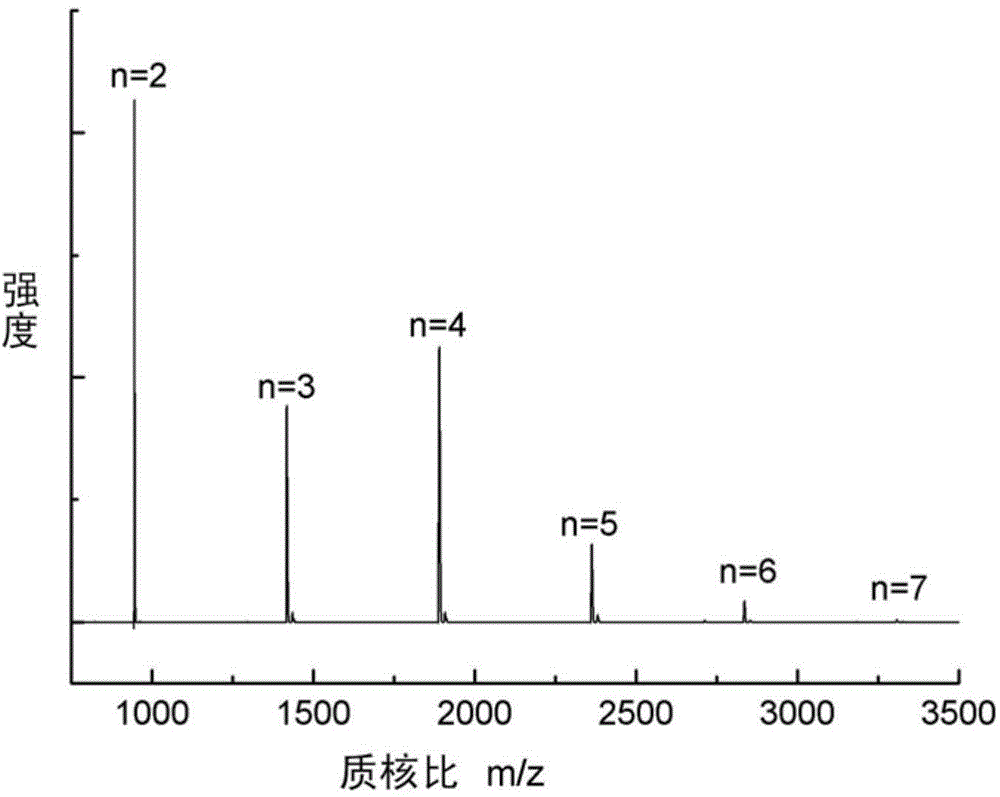
[0033] The R 1 Is phenyl, substituted phenyl or C1-C10 alkyl, said R 2 It is a C1-C10 alkylene group or a bisphenol compound after losing two hydroxyl groups, n is the degree of polymerization, and n=2-7.
[0034] In the present invention, the compound having the structure of formula (II) is The R 1 It is a phenyl group, a substituted phenyl group, or a C1-C10 alkyl group, preferably a methyl group, a phenyl group, or a phenoxy group, and more preferably a phenyl group. That is, the compound having the structure of formula (II) is preferably phenylpho...
Embodiment 1
[0056] Weigh 7mmol of phenylphosphonic dichloride and dissolve in 50ml of dichloromethane to obtain a dichloromethane solution of phenylphosphonic dichloride. Weigh 7mmol of bisphenol A monomer and dissolve in 50ml of 0.28mol / L sodium hydroxide aqueous solution In the process, an aqueous sodium hydroxide solution of bisphenol A monomer is obtained.
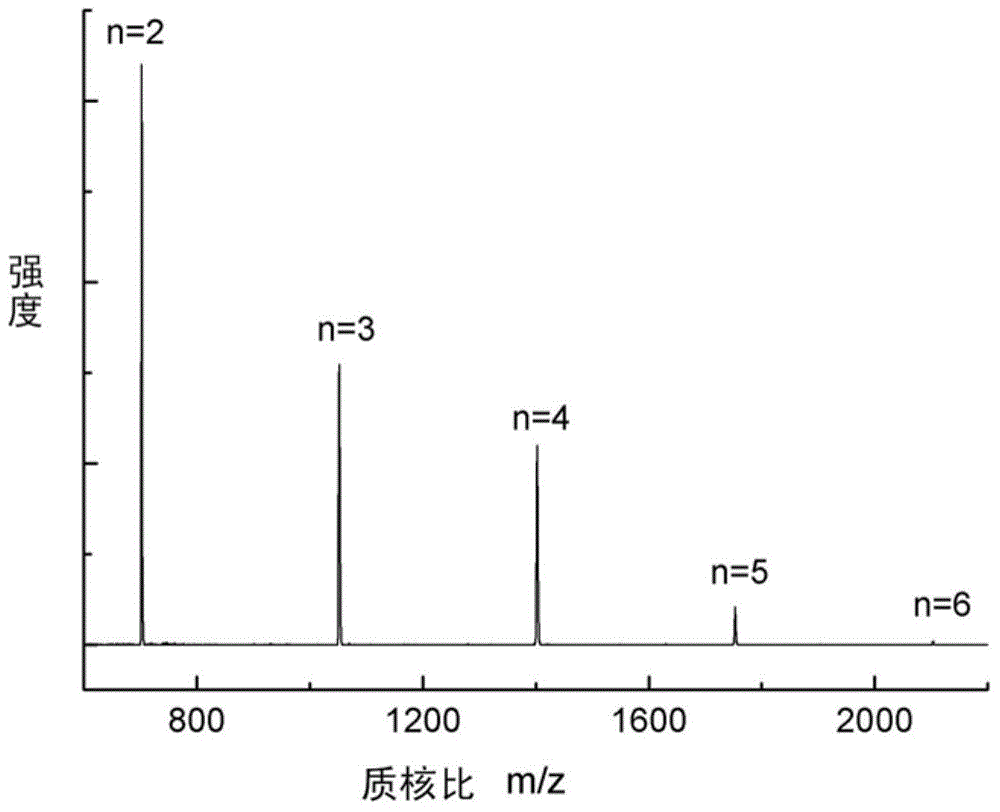
[0057] Add the above two solutions slowly and uniformly within 10 hours to a 500ml four-neck flask filled with 200ml dichloromethane, 40ml deionized water and 0.2g cetyltrimethylammonium bromide under vigorous stirring In, and access to N 2 Protected, the reaction temperature is controlled at -20°C-20°C, and the reaction is continued for 3 hours after the addition is completed. After the reaction, the organic phase was separated with a separatory funnel, washed 2-3 times with deionized water to remove inorganic salts, and the solvent was evaporated to obtain a white solid product, namely 2.1 g of cyclic polyphosphate oligomer, with a...
Embodiment 2
[0065] Weigh 7mmol of phenylphosphonic dichloride and dissolve it in 50ml of dichloromethane to obtain a dichloromethane solution of phenylphosphonic dichloride. Weigh 7mmol of phenolphthalein monomer and dissolve it in 50ml of 0.28mol / L sodium hydroxide aqueous solution. A sodium hydroxide aqueous solution of phenolphthalein monomer was obtained.
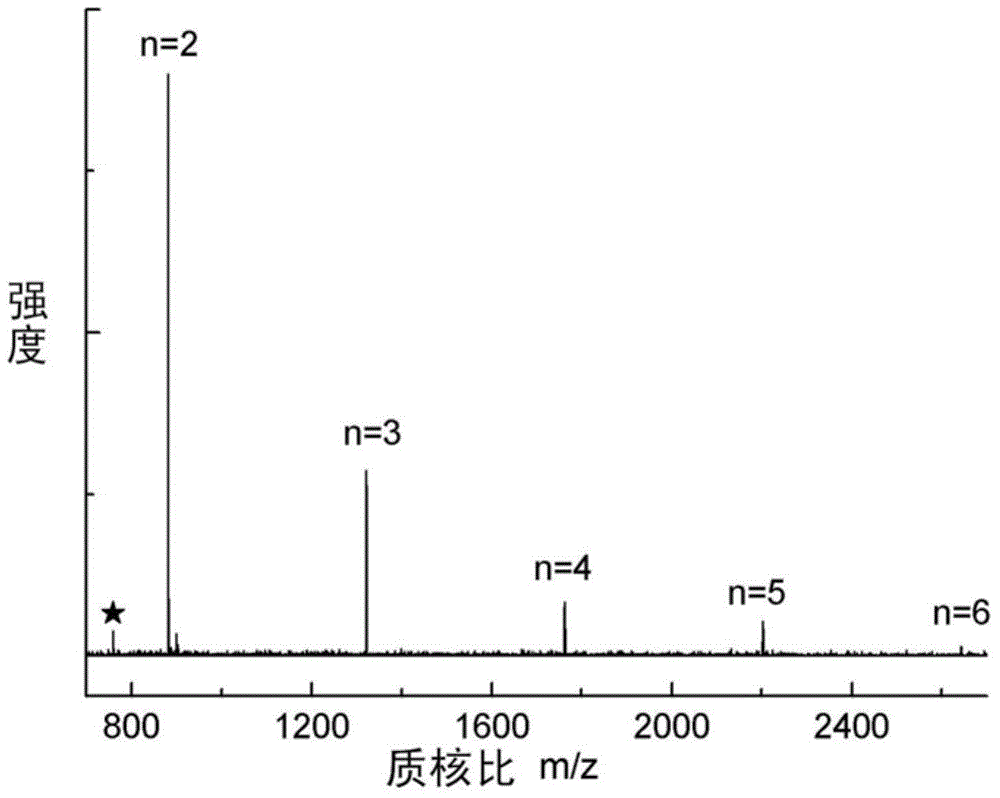
[0066] Add the above two solutions slowly and uniformly within 10 hours to a 500ml four-neck flask filled with 200ml dichloromethane, 40ml deionized water and 0.2g cetyltrimethylammonium bromide under vigorous stirring In, and access to N 2 Protected, the reaction temperature is controlled at -20°C-20°C, and the reaction is continued for 3 hours after the addition is completed. After the reaction, the organic phase was separated with a separatory funnel, washed 2-3 times with deionized water to remove inorganic salts, and the solvent was evaporated to dryness to obtain a white solid product, namely 2.22 g of cyclic polyphosphate oligo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com