Supported nickel-iron composite hydroxide oxygen evolution electrode for alkaline water electrolysis and preparation method for supported nickel-iron composite hydroxide oxygen evolution electrode
A composite hydroxide and oxygen evolution electrode technology, applied in the direction of alkaline battery electrodes, battery electrodes, circuits, etc., can solve the problems of low raw material utilization rate, complicated process, time-consuming, etc., and achieve simple and effective process and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
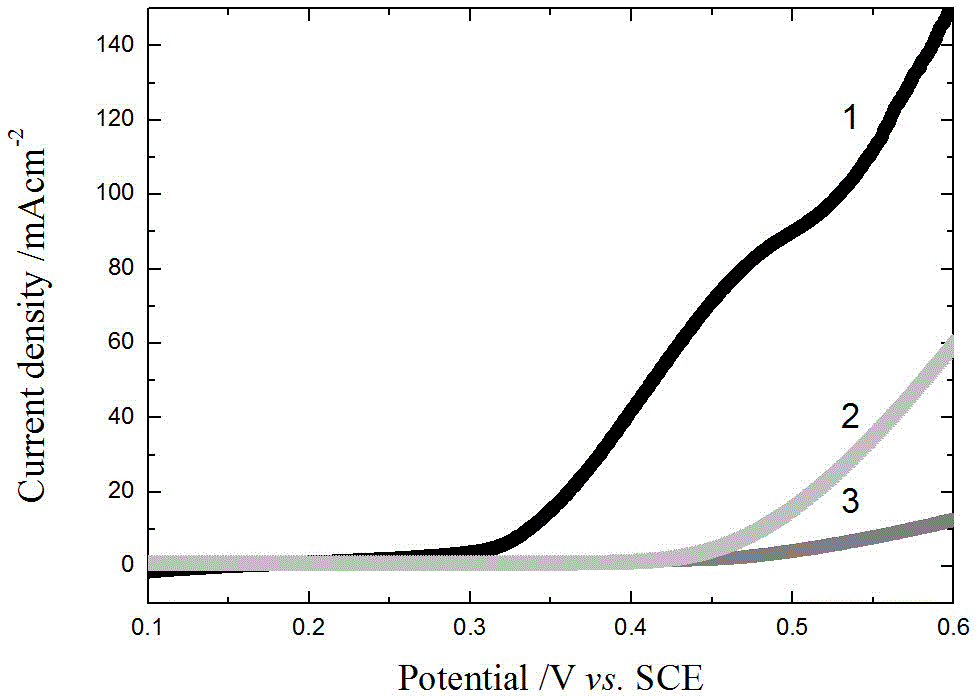
Image
Examples
Embodiment 1
[0023] 1g nickel nitrate hexahydrate, 0.4g ferric nitrate nonahydrate, 0.8g PTFE emulsion, and 1g conductive acetylene black were sequentially added to 4ml water and ethanol mixed solvent. Then use a glass rod to stir evenly and wait for a small amount of water and ethanol to volatilize to form a paste. This paste is rolled on a roller press into a NiFe with a thickness of 200 μm x salt / C composite film, put the film in an oven to heat up to 120 ℃, heat treatment in air atmosphere for 1 h to obtain NiFe x salt / C Composite membrane. This NiFe x salt The / C composite membrane was placed in 3 M KOH solution for in-situ precipitation reaction at room temperature for 3 hours. NiFe deposited in situ x (OH) y / C composite film is washed and dried, and cut into NiFe with a size of 5cm*6cm x (OH) y / C sheet, then press the sheet on the foamed nickel after degreasing and drying under 10 Mpa pressure to obtain NiFe x (OH) y / C / M oxygen evolution electrode.
Embodiment 2
[0025] In this example, NiFe is obtained x salt The process of the / C composite membrane is the same as in Example 1, except that the metal current collector is pressed first, and then the in-situ precipitation reaction is performed. Upcoming NiFe x salt / C composite film is cut into a size of 5cm*6cm, and pressed on the degreasing and dried nickel foam collector under a pressure of 5Mpa, and then NiFe x salt / C / M composite membrane is placed in 3 M KOH solution for in-situ precipitation reaction at room temperature for 3 hours to obtain NiFe x (OH) y / C / M oxygen evolution electrode.
Embodiment 3
[0027] 3g nickel nitrate hexahydrate, 3g ferric nitrate nonahydrate, 3g PTFE emulsion, 6g Cabot conductive carbon black were sequentially added to 8ml water and ethanol mixed solvent. Then use a glass rod to stir evenly and wait for a small amount of water and ethanol to volatilize to form a paste. This paste is rolled on a roller press into a NiFe with a thickness of 240 μm x salt / C composite film, the film is heated to 200 ℃ in a muffle furnace, heat treatment in air atmosphere for 0.5 h to obtain NiFe x salt / C Composite membrane. This NiFe x salt The / C composite membrane was placed in a 7 M KOH solution for in-situ precipitation at room temperature for 30 minutes. NiFe deposited in situ x (OH) y / C composite film is washed and dried, and then cut into NiFe with a size of 10 cm*10 cm x (OH) y / C sheet, then press the sheet on the degreasing and drying stainless steel net under 20 Mpa pressure to obtain NiFe x (OH) y / C / M oxygen evolution electrode.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com