Pharmaceutical composition for treating anaphylactoid purpura and preparation method of pharmaceutical composition
A technology for allergic purpura and a composition, which is applied in the field of traditional Chinese medicine and can solve the problems of easy recurrence and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
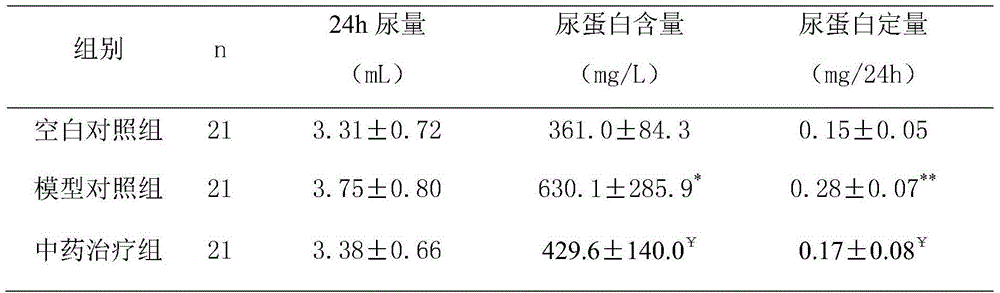
Examples
Embodiment 1
[0018] The preparation of embodiment 1 Chinese medicine extract
[0019] (1) Ingredients: Desmodium 250g, Plantago 250g, Haijinsha 140g, Corn silk 140g, Hedyotis diffusa 140g, Houttuynia cordata 140g, Imperata 140g, bell bean 140g, Salvia 140g, safflower 100g, peach kernel 100g, Eupatorium 100g, Qumai 100g, Motherwort 100g, Spatholobus 100g, Chinese rose 140g, pearl ginseng 140g, paeonol 140g, scrophulariaceae 140g, red peony 140g, comfrey 140g, Baiwei 140g, rehmannia 140g, ink Eclipta 140g, Anemarrhena 140g, Scutellaria baicalensis 100g, Forsythia 100g, Quanshen 100g;
[0020] (2) Take paeonol root, salvia miltiorrhiza, rehmannia root of prescription quantity, mix, pulverize, add volume concentration and be 70% ethanol solution diafiltration, the volume of ethanol solution is 12 times of medical material quality, leachate decompression is recovered to no alcohol Taste, concentrate, get percolation concentrate and medicinal residues for later use;
[0021] (3) Mix the dregs ...
Embodiment 2
[0023] The preparation of embodiment 2 Chinese medicine granules, capsules
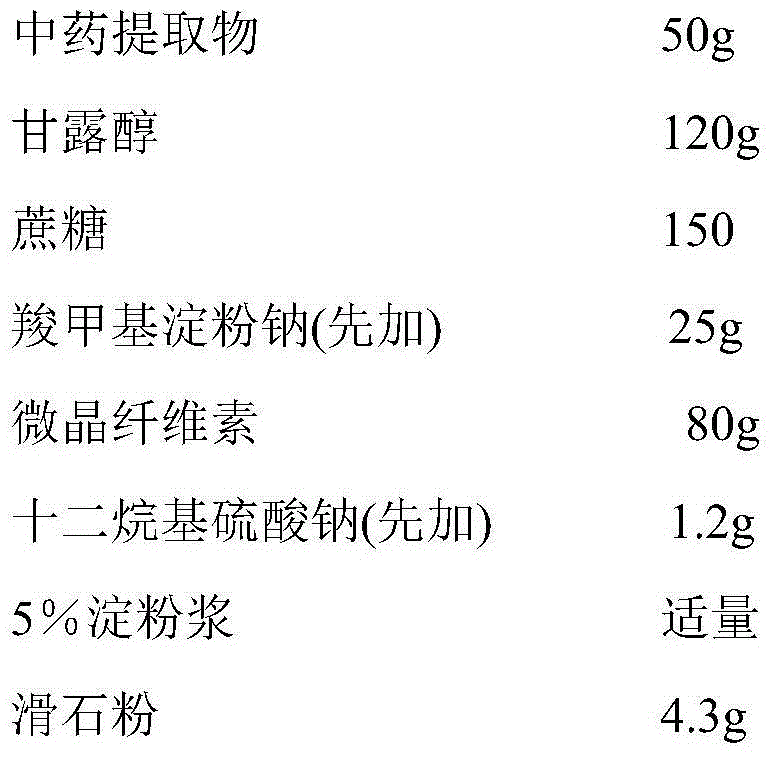
[0024] prescription:
[0025]
[0026]Preparation process: pass mannitol, sucrose, sodium carboxymethyl starch, microcrystalline cellulose, and sodium lauryl sulfate through a 100-mesh sieve, and set aside; weigh the dry powder of the Chinese medicine extract prepared in Example 1, and mannitol, sucrose , microcrystalline cellulose, sodium carboxymethyl starch, sodium lauryl sulfate, mix well, add 5% starch slurry to make soft material, pass through 20 mesh sieve to make wet granules, dry at 50-60°C for 2 hours, pass through 16 mesh Sieve the granules, add talcum powder, and mix well; divide into 20g / bag to obtain granules, or fill capsule shells to obtain capsules.
Embodiment 3
[0027] The preparation of embodiment 3 Chinese medicine tablet
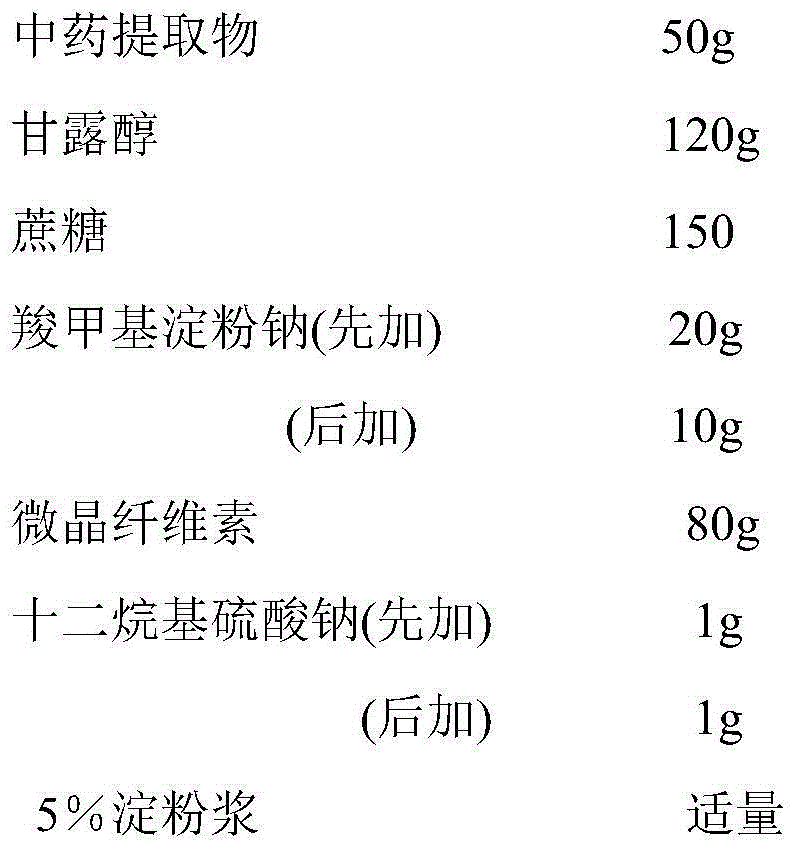
[0028] prescription:
[0029]
[0030] Preparation process: pass mannitol, sucrose, sodium carboxymethyl starch, microcrystalline cellulose, and sodium lauryl sulfate through a 100-mesh sieve, and set aside; weigh the dry powder of the Chinese medicine extract prepared in Example 1, and mannitol, sucrose , microcrystalline cellulose, 2 / 3 amount of sodium carboxymethyl starch, 1 / 2 amount of sodium lauryl sulfate, mix well, add 5% starch slurry to make soft material, pass through a 20-mesh sieve to make wet granules, 50 Dry at ~60°C for 2 hours, pass through a 16-mesh sieve for granulation, add the remaining 1 / 3 amount of sodium carboxymethyl starch and 1 / 2 amount of sodium lauryl sulfate, mix well, press 1000 tablets, each tablet contains Chinese herbal extract 50mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com