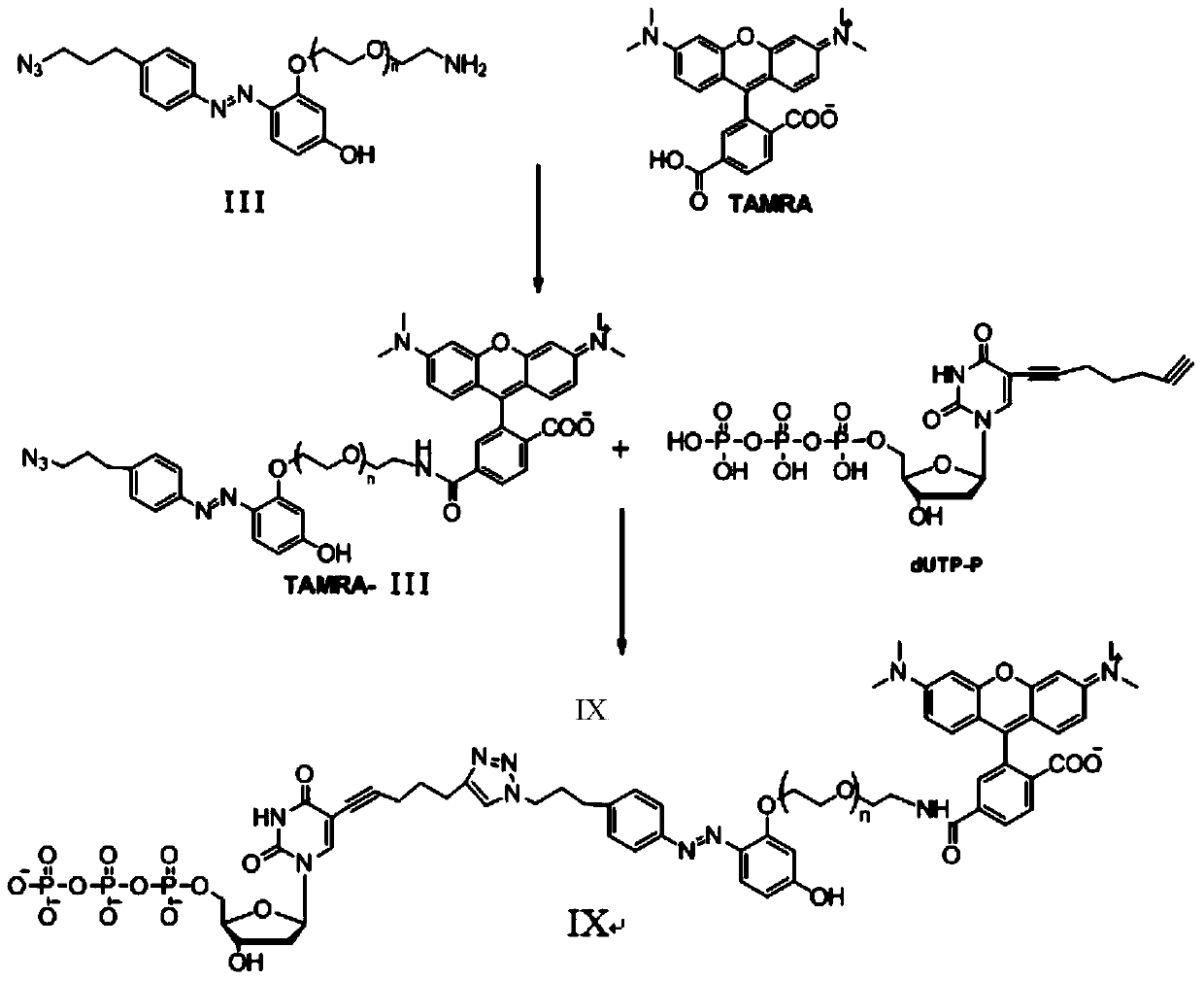
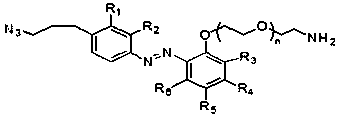
Azo linkage unit based fluorescence labeled nucleotide and applications thereof
A ligation unit and fluorescent labeling technology, applied in the field of DNA sequencing, can solve the problems of mild shearing conditions, low efficiency, short read length, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
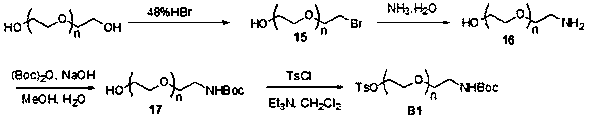
[0072] Example 1. Synthesis of intermediate compound B1
[0073] The synthesis schematic diagram of the intermediate compound B1 of this embodiment is as follows: figure 2 As shown, the specific steps are as follows:
[0074] (1) Synthesis of compound 15: dissolve polyethylene glycol (9g, 60mmol) in 70mL of toluene, then add 10.2mL of 48% HBr aqueous solution, heat to reflux, absorb the emitted gas with sodium bicarbonate, and control the reaction temperature to The reaction was stirred at 115°C for 3 days. After cooling the reaction solution, add saturated sodium bicarbonate aqueous solution to make it neutral, then spin off the solvent, add 30mL water, and use CH 2 Cl 2 Extract (3*60mL). Combine the organic phases, dry with anhydrous sodium sulfate, and spin off the organic solvent to obtain 5.2 g of compound 15 with a yield of 41%. 1 H NMR(400MHz, CDCl 3 ): δppm 3.68(t,2H,J=6.0Hz), 3.58(t,2H,J=4.6Hz), 3.51-3.55(m,4H), 3.44(t,2H,J=4.6Hz), 3.35( t, 2H, J = 6.3 Hz).
[0075] (2)...
Embodiment 2
[0078] Example 2. Synthesis of intermediate compounds A31 and A71
[0079] The synthesis schematic diagram of the intermediate compounds A31 and A71 in this example is as follows: image 3 As shown, the specific steps are as follows:
[0080] (1) Synthesis of compound 2: 4-iodoaniline (4.38g, 20mmol) was dissolved in 30ml ethyl acetate, 2ml of triethylamine was added, benzyl chloroformate (3.4g, 20mmol) was added dropwise under ice water bath, and stirred to The reaction was carried out at room temperature for 4 hours, the aqueous phase of the reaction liquid, the organic phase were dried with anhydrous sodium sulfate, and the solvent was evaporated to dryness to obtain 27.27 g of the product compound with a yield of 97%. 1 H NMR(500MHz, CDCl 3 )δ7.89–7.71(m,4H), 7.40–7.24(m,5H), 7.10(s,1H), 4.65(s,2H);
[0081] (2) Synthesis of compound A11: Add compound 2 (0.7mmol, 247mg) into a single-necked flask, then weigh 9.7mg CuI and 20.3mg Pd(PPh 3 ) 4 Add to the reaction flask, vacuum, ni...
Embodiment 3
[0089] Example 3. Synthesis of intermediate compounds B11 and B12
[0090] The synthesis schematic diagram of the intermediate compounds B11 and B12 in this example is as follows: Figure 4 As shown, the specific steps are as follows:
[0091] (1) Synthesis of compound B11: Dissolve compound phloroglucinol (47mg, 0.372mmol) and potassium carbonate (19mg, 0.136mmol) in 3mL DMF in a 10mL single-neck flask, and dissolve compound B1 (50mg, 0.124mmol) Add 3ml DMF to the reaction flask, and stir at 120°C for 2.5h under nitrogen protection. After cooling to room temperature, 20 mL of ethyl acetate was added, and then washed with water, dried over anhydrous sodium sulfate, and the organic solvent was spun off. The residue was separated by column chromatography to obtain 27 mg of compound B11 with a yield of 59%. 1 H NMR(500MHz, CDCl 3 )δ6.00–5.87(m,3H),4.72(brs,2H),4.59(s,1H), 4.30(s,1H), 3.76(s,1H), 3.66(t,J=8.1Hz,2H ),3.51(s,4H),3.04(t,J=8.1Hz,2H),1.42(s,9H). 13 C NMR(125MHz, CDCl 3 )δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com