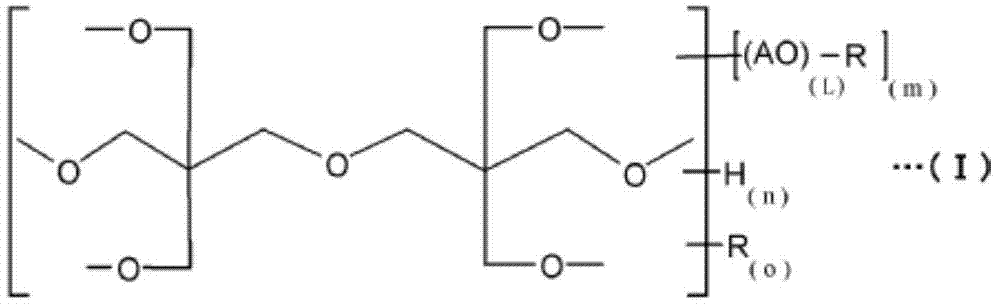
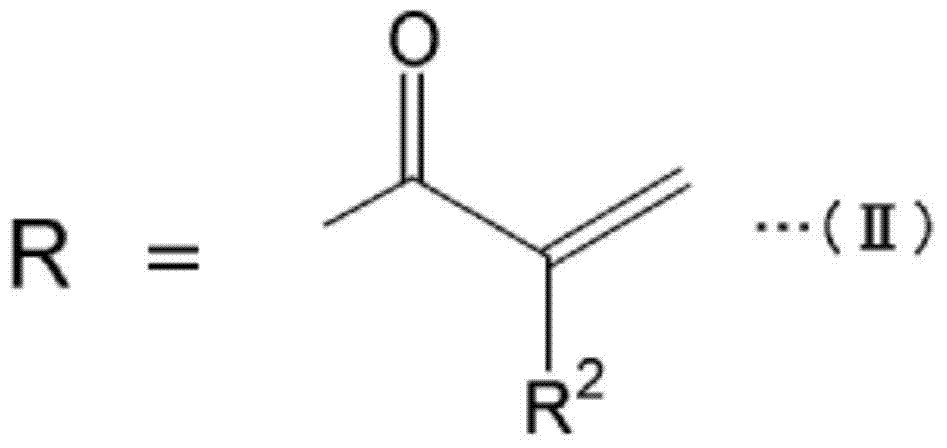
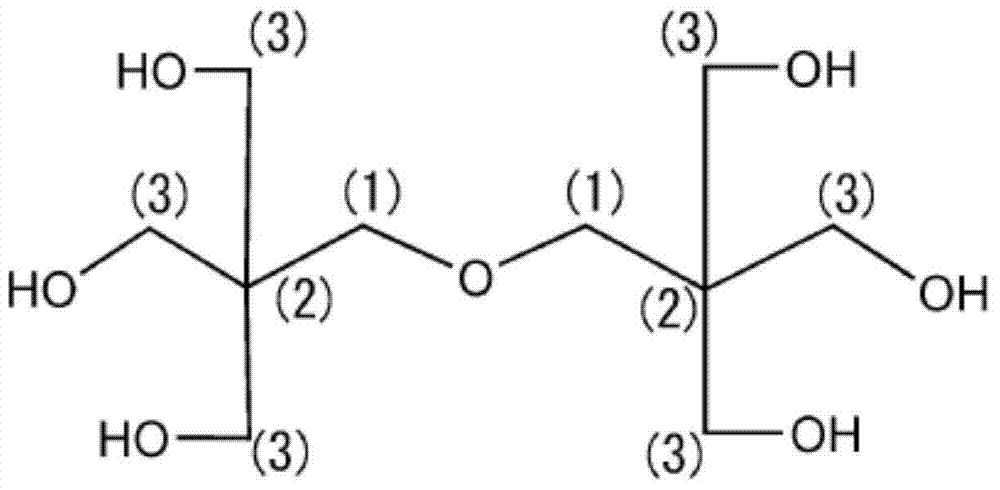
Urethane acrylate, and reactive composition containing same
A technology of urethane acrylate and acrylate, which is applied in the field of urethane acrylate, can solve the problems of high viscosity and reduced adhesion, and achieve the effects of low viscosity, improved adhesion and high crosslinking density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0062] The present invention will be described in more detail with reference to the following examples. Insofar as it exceeds the spirit of its scope, the present invention is not limited by any of the following examples. Unless otherwise specified, "%" is % by mass, and "part" is part by mass.
[0063]
[0064] In Examples and Comparative Examples, the conditions used for LC-MS are as follows.
[0065] [LC part] Manufactured by Agilent Technologies, 1100Series,
[0066] Column: Inertsil ODS-2 (4.6mmφ×250mm, 5μm),
[0067] Eluent: water 80.0%-30min→0.0%, methanol 20.0%-30min→100.0%,
[0068] Column temperature: 40°C,
[0069] Flow rate: 1mL / min, injection volume: 5μL (200ppm methanol solution),
[0070] Detectors: UV, RI.
[0071] [MS part] JMS T100LP (manufactured by JEOL)
[0072] Ring lens voltage: 10V, ionization method: APCI+, solvent removal chamber temperature: 350°C,
[0073] Needle voltage: 2500V, orifice 1 temperature: 80°C, orifice 1 voltage: 60V,
[0074...
Synthetic example 1
[0082] (Synthesis of dipentaerythritol 2EO adduct acrylate)
[0083] 254 g (1.0 mol) of dipentaerythritol (manufactured by Koei Chemical Company, OH value 1324), 127 g of toluene and 0.3 g of KOH were placed in an autoclave having a volume of 1 L and equipped with a stirrer, heated to 90° C. and stirred, to obtain a suspension. Next, it was heated to 130° C., and 132 g (3 mol) of ethylene oxide was gradually introduced into the autoclave and reacted. With the introduction of ethylene oxide, the internal temperature of the autoclave increased. It was cooled as necessary to keep the reaction temperature below 140°C. After the reaction, it was depressurized at 140° C. under a mercury column of 10 mmHg or less, thereby removing excess ethylene oxide and by-product ethylene glycol polymer. Subsequently, it was neutralized with acetic acid to obtain an adjusted pH of 6-7. The dipentaerythritol 2EO adduct obtained (average 2 mol of added ethylene oxide—the same applies below) had...
Synthetic example 2
[0095] (Synthesis of dipentaerythritol 4EO adduct acrylate)
[0096] 254 g (1.0 mol) of dipentaerythritol (manufactured by Koei Chemical Company, OH value 1324), 127 g of toluene and 0.3 g of KOH were placed in an autoclave having a volume of 1 L and equipped with a stirrer, heated to 90° C. and stirred, to obtain a suspension. Next, it was heated to 130° C., and 220 g (5 mol) of ethylene oxide was gradually introduced into the autoclave and reacted. With the introduction of ethylene oxide, the internal temperature of the autoclave increased. It was cooled as necessary to keep the reaction temperature below 140°C. After the reaction, it was decompressed at 140° C. to a mercury column of 10 mmHg or less, thereby removing excess ethylene oxide and by-product ethylene glycol polymer. Subsequently, it was neutralized with acetic acid to obtain an adjusted pH of 6-7. The OH value of the obtained dipentaerythritol 4EO adduct was 765.
[0097] Put ethylene glycol modified dipent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com