Stichopus japonicas BPI gene, encoded protein, cloning method of stichopus japonicas BPI gene, and method for constructing recombinant stichopus japonicas BPI genetically engineered bacterium
A technology of genetic engineering bacteria and cloning method, which is applied in the field of coding protein and its cloning, sea cucumber BPI gene, and construction of recombinant sea cucumber BPI genetic engineering bacteria, and can solve problems such as antibiotic failure, sea cucumber stimulation, and bacterial resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0051] BPI gene cloning and sequence analysis
[0052](1) Through high-throughput transcriptome sequencing and expression profiling analysis of coelomocytes of sea cucumbers with rot skin syndrome and healthy sea cucumbers, multiple EST sequences encoding BPI genes were found (Pengjuan Zhang, Chenghua Li, Lin Zhu, Xiurong Su, Ye Li, Chunhua Jin, Taiwu Li. De Novo assembly of the sea cucumber Apostichopus japonicus hemocytes transcriptome to identify miRNA targets associated with skin ulceration syndrome. Plos ONE 2013. 8(9):e73506.), select EST clones encoding partial fragments of A. japonicus BPI;
[0053] (2) RACE primer design: Design nested primers for RACE based on EST clones encoding partial fragments of A. japonicus: 3' upstream specific primer 1: TTCAAAGCAAAAACAACCCGTC, 3' upstream specific primer 2: TGGGTGTCATCTTTTGAAGGTGT; 5' downstream specific primer 1: GCACTGTTGATGAGGTAGTCGCT, 5' downstream specific primer 2: GTGTCCGCAGTAAGGAGTAATCT, amplified 3' adapter pri...
specific Embodiment 2
[0061] Construction Method of Recombinant Apostichopus japonicus BPI Genetic Engineering Bacteria
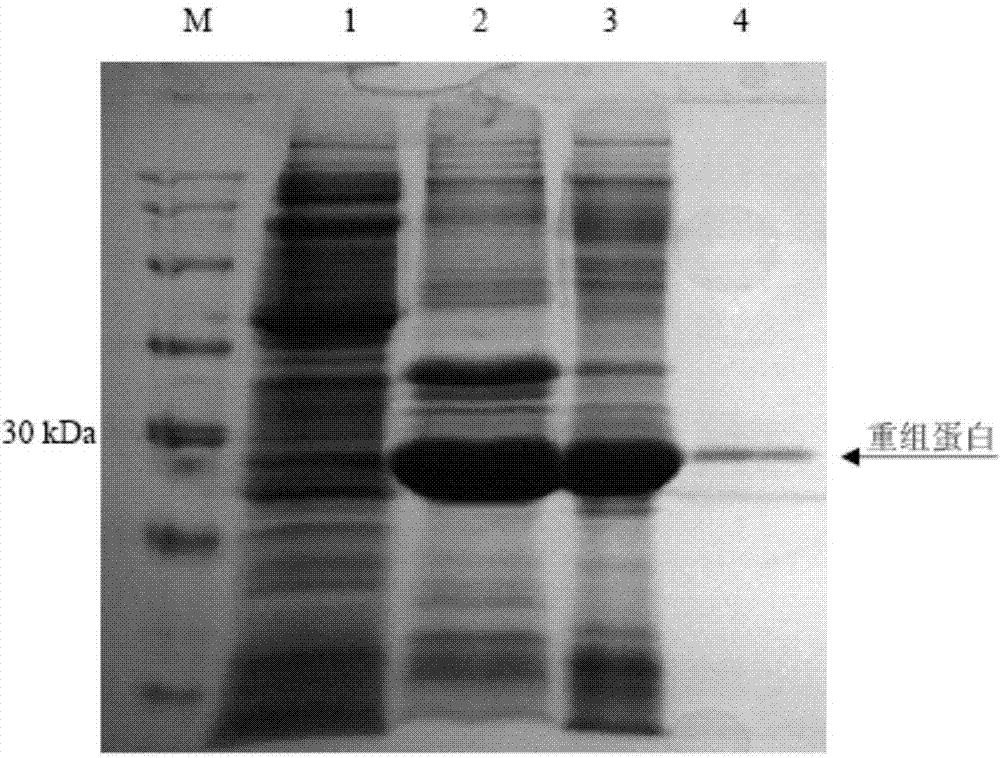
[0062] 1. Cloning of N-terminal domain of BPI protein and construction and expression of recombinant protein
[0063] a. Extraction of total RNA: Take 1.0 mL of body cavity fluid of sea cucumber, centrifuge at 800 g for 5 min, collect blood cells, add 1.0 mL of Trizol reagent (purchased from Takara Company), vortex and mix well, leave at room temperature for 5 min, then add 0.2 mL of chloroform, Shake and mix well, let stand at room temperature for 10 minutes, centrifuge at 12,000 g for 15 minutes at 4°C, pipette the supernatant into a centrifuge tube, add an equal volume of isopropanol to the supernatant, mix well, let stand at room temperature for 5 minutes, 4°C , 12,000 rpm, centrifuge for 10 min, remove the supernatant, add 1 mL of ethanol with a mass percentage concentration of 75% to the pellet, centrifuge at 4°C, 12,000 rpm for 5 min, remove the supernatant, and let t...
specific Embodiment 3
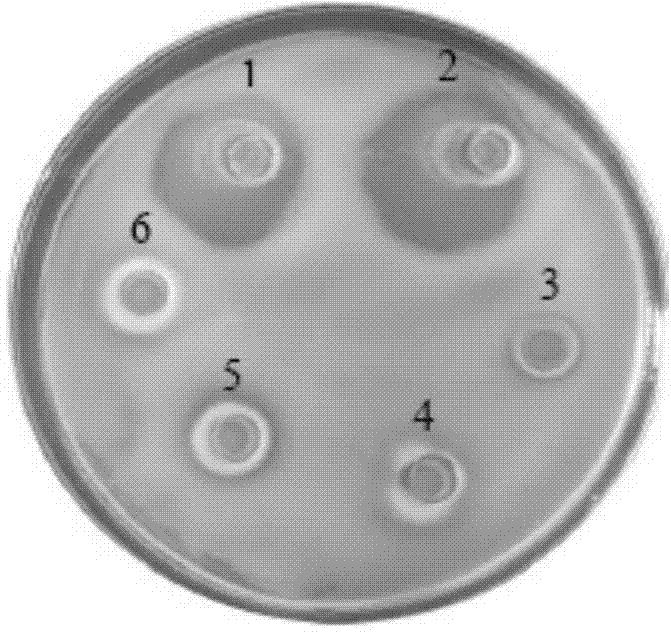
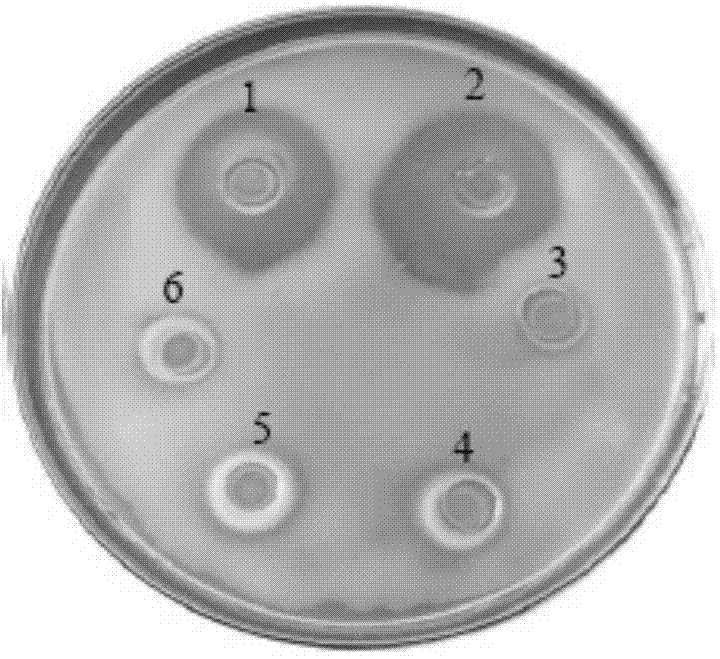
[0081] Antibacterial activity analysis of recombinant protein of Apostichopus japonicus
[0082] (1) Four test-negative bacteria Vibrio parahaemolyticus ( Vibrio parahaemolyticus ), Vibrio harveii ( Vibrio Harvey ), Vibrio splendidus ( Vibrio splendidus ), Vibrio alginolyticus (Vibrio alginolyticus) were inoculated in 2216E liquid medium (tryptone 5g / L, yeast extract 1 g / L, pH=7.6) at 28 ℃, 150 r / min and cultivated to OD 600 =1.0, take 100 ul of bacterial solution and smear the plate (agar 12 g / mL);
[0083] (2) The tested positive bacteria Micrococcus luteus ( Micrococcus luteus ) inoculated in nutrient agar liquid medium (tryptone 10 g / L, beef extract 3 g / L, NaCl 5g / L, pH=7.3±0.1) and cultivated at 35 °C and 150 r / min. Solid medium (agar 15 g / L) was mixed and poured on the plate, the concentration of bacteria was 1×10 7 cfu / mL;
[0084] (3) Use the modified agar plate diffusion method (Oxford cup) to measure the antibacterial activity of the recombinant bactericida...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com