A kind of di(benzothiophene)ethylene polymer and its preparation method and application
A technology of benzothiophene and polymers, applied in the application field of diethylene polymers and its preparation, and field effect transistors, can solve the problems of less research on materials, achieve good stability, good application prospects, and simple synthetic routes Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
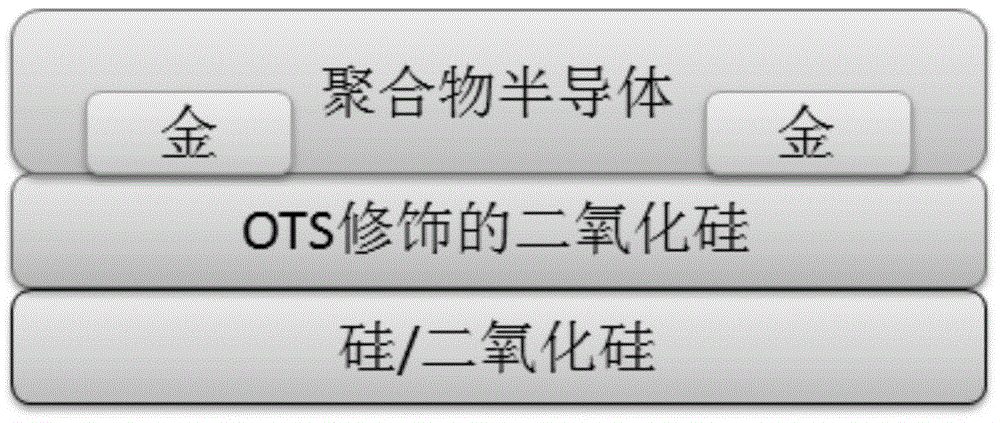
Image
Examples
Embodiment 1
[0061] Example 1 Synthesis of (n=12, P6TBE-8-10)
[0062] 1) Synthesis of (E)-1,2-bis([1]benzothiophen-6-yl)ethylene
[0063] Titanium tetrachloride (1.6mL, 13.9mmol, 1.2eq) was added dropwise to anhydrous tetrahydrofuran (50mL) cooled in an ice-salt bath under a nitrogen atmosphere, and after stirring for 30 minutes, Zn powder (1.9g) was added, and the reaction mixture was Heat and boil for 1h. After cooling down in an ice bath, benzothiophene-6-carbaldehyde (1.88 g, 11.6 mmol) was added dropwise, stirred for 15 min, and then the reaction solution was heated to boiling for 4 h. After cooling to room temperature, the reaction was quenched with 100 mL of ice water, filtered, and the filter cake was washed with ethanol and then recrystallized with toluene to obtain 1.21 g of light yellow crystals. Yield: 71.3%. Structural characterization data are as follows:
[0064] Mass Spectrum: [MS(EI)]m / z:292(M + ), [HR-EI] 292.0380.
[0065] H NMR and C NMR: 1 H NMR (300MHz, CDCl ...
Embodiment 2
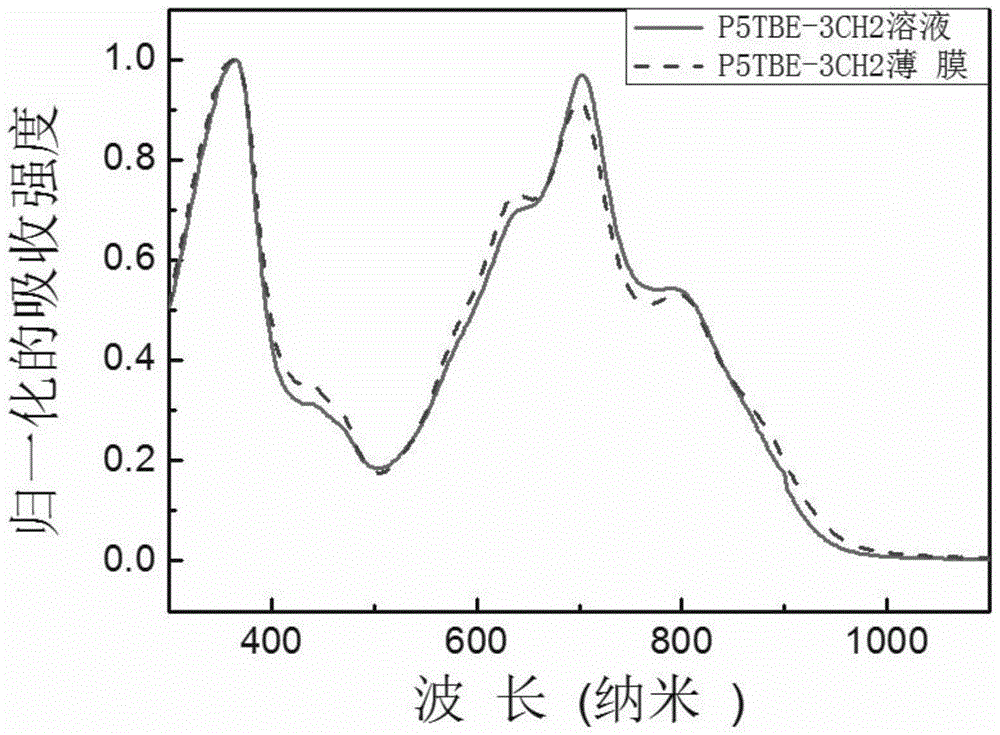
[0077] Embodiment 2, Synthesis of (n=17, P5TBE-8-10)
[0078] 1) Synthesis of (E)-1,2-bis([1]benzothiophen-5-yl)ethylene
[0079] Titanium tetrachloride (3.2mL, 27.8mmol, 1.5eq) was added dropwise to anhydrous tetrahydrofuran (50mL) cooled in an ice-salt bath under a nitrogen atmosphere, and after stirring for 30 minutes, Zn powder (3.4g, 2.8eq) was added , The reaction mixture was heated and boiled for 1h. After cooling down in an ice bath, benzothiophene-5-carbaldehyde (3.00 g, 18.5 mmol) was added dropwise, stirred for 20 min, and then the reaction solution was heated to boiling for 6 h. After cooling to room temperature, the reaction was quenched with 100 mL of ice water, filtered, and the filter cake was washed with ethanol and then recrystallized with toluene to obtain 1.87 g of white crystals. Yield: 69%. Structural characterization data are as follows:
[0080] Mass spectrum: [HR-EI] 292.0380.
[0081] H NMR and C NMR: 7.942-7.939 (m, 2H), 7.868 (d, 2H, J = 8.1Hz...
Embodiment 3
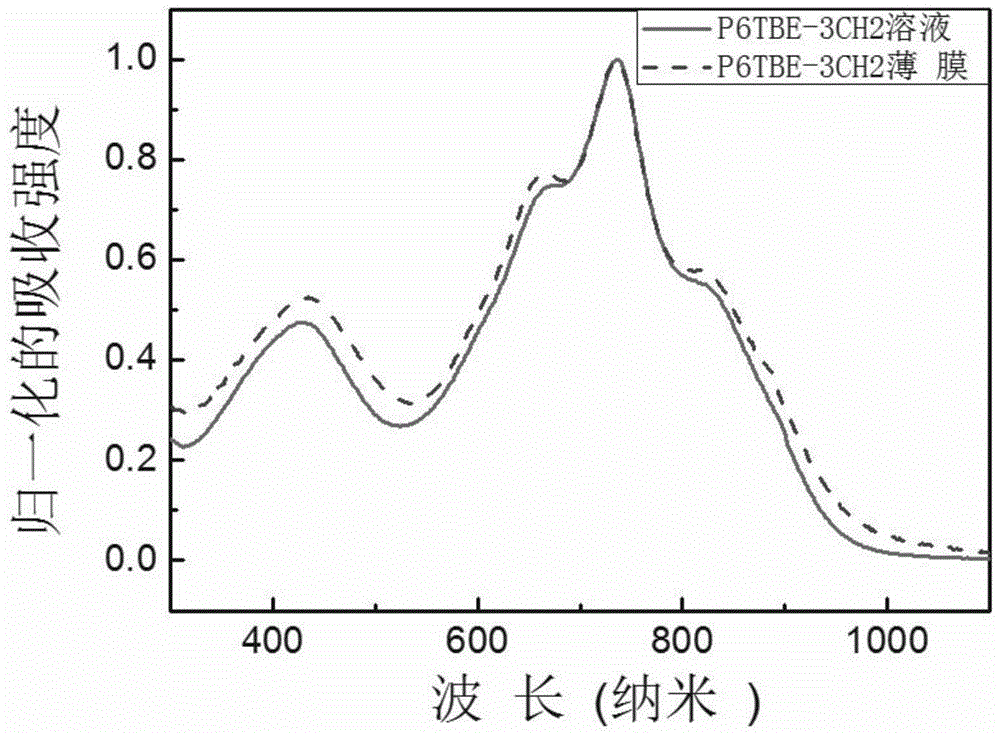
[0092] Embodiment 3, Synthesis of (n=21, P6TBE-3CH2)
[0093] 1) Synthesis of compound a
[0094] Into a 500mL single-necked bottle, add 8.4g 3,6-bis(2-thienyl)pyrrolo[3,4-c]pyrrole-1,4-dione in sequence (refer to literature Li, Yuning; Sonar, Prashant; Singh, Samarendra P.; Soh, Mui Siang; VanMeurs, Martin; Tan, Jozel, J. Am. Chem. Soc., 2011, 133, 2198–2204 Synthesis), anhydrous potassium carbonate 9.0 g and 90 mL of DMF. Under the protection of nitrogen, after stirring at 100°C for 60min, 30.7g of 1-iodo-4-decyltetradecane and 40mg of 18-crown-6 were added, and then stirring was continued at 120°C for 24h. Extract with chloroform and dry over anhydrous magnesium sulfate. The crude product was sonicated with methanol to wash away a small amount of large polar impurities, and then sonicated with a small amount of n-hexane to remove excess iodide. Afterwards, the residue was separated by silica gel column chromatography (dichloromethane:petroleum ether=0-0.5) to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com