Method for preparing polyurethane hydrogel responsive to both light and reducing agent
A responsive, hydrogel technology, used in bandages, absorbent pads, medical science and other directions, can solve the problems of complex preparation methods of light-responsive hydrogels, low strength, and few research reports on light-responsive polyurethane hydrogels. Achieving the effects of favorable regulation, low cost and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the prepolymer is as follows: Weigh 8.0~9.0g polyethylene glycol and 0.88~1.2mL hexamethylene diisocyanate into N 2 In a three-necked flask under protection, weigh 40-80mL of anhydrous N,N-dimethylformamide into the three-necked flask, dissolve PEG and HDI under magnetic stirring, heat the system to 80-90℃, and drop 100 ~200μL of stannous octoate catalyst, react for 2~4 hours to obtain prepolymer;
[0035] The preparation method of the crosslinked network is as follows: Weigh cyclodextrin (0.05~0.50g), azobenzene (0.008~0.08g) and DSDPDO (0.05~0.50g) in a beaker, add 10-15mL anhydrous N,N -Dimethylformamide is dissolved, the solution is added to the prepolymer prepared in the above steps, mixed thoroughly and poured into a mold, the mold is heated to 80-90°C, the sample is cured for 18-36 hours, and the polymer is cross-linked The internet;
[0036] The post-treatment method is as follows: After the cross-linked network is soaked in 300-500mL metha...
Embodiment 1
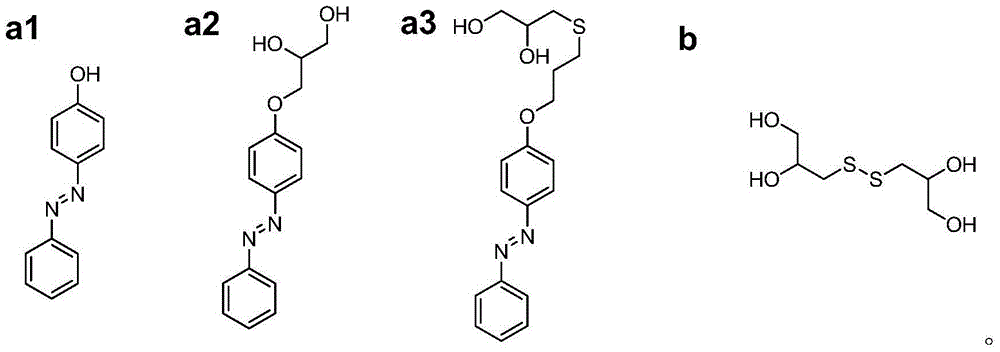
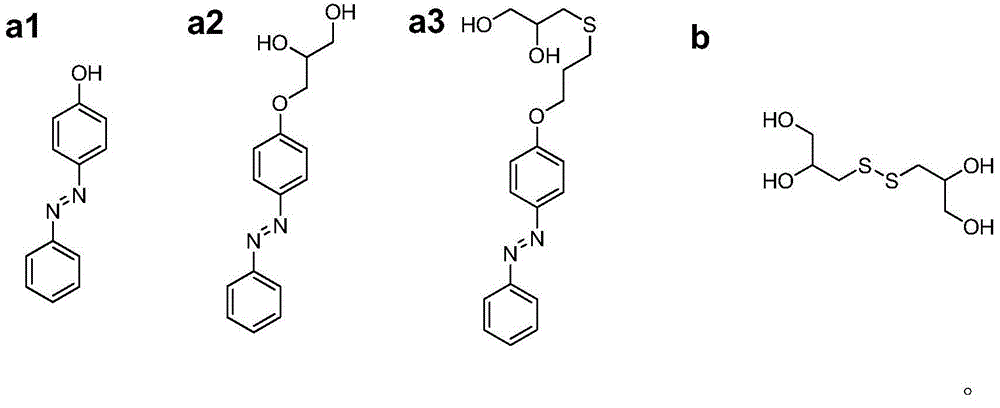
[0041] Thioglycerin (12g, 100mmol) was dissolved in 20mL of methanol, and hydrogen peroxide (6.8g, 60mmol, 30wt% aqueous solution) was added dropwise to it, and after the addition was completed, it was stirred at room temperature for 6h. The solvent was removed by rotary evaporation to obtain a colorless transparent viscous liquid. The crude product was recrystallized in a mixed solvent of n-hexane / methanol to obtain a white solid DSDPDO (10.0 g, yield 93.2%).
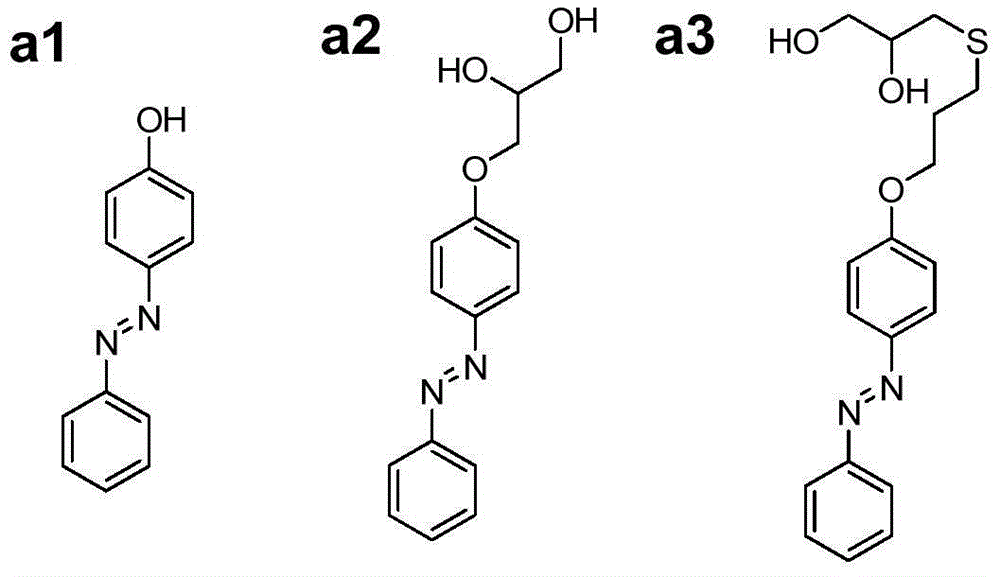
[0042] Dissolve 8.5g polyethylene glycol (molecular weight 10000g / mol, PDI=1.1) and 0.914mL hexamethylene diisocyanate in 40mL anhydrous N,N-dimethylformamide. After heating to 85℃, drop in 140μL of stannous octoate catalyst was reacted for 3 hours to obtain prepolymer; Weigh β-cyclodextrin (0.25g), 4-phenylazophenol (0.044g) and DSDPDO (0.25g) and dissolve in 12mL anhydrous N ,N-Dimethylformamide, add the solution to the prepolymer prepared in the above steps, mix well, pour it into the mold, heat to 85°C and cure for 24...
Embodiment 2
[0044] The preparation method of dithioglycerol (3,3'-disulfane disubstituted dipropyl-1,2-diol, DSDPDO) is the same as in Example 1.
[0045] Weigh 4-phenylazophenol (4g, 20mmol) and potassium carbonate (8.29g, 60mmol) and dissolve them in 50mL of acetone. 2 Add allyl bromide (4.8g, 40mmol) under protection, heat the system to acetone reflux, react for 24h and then cool to room temperature. The product was filtered to obtain a filtrate. After concentration, the crude product was recrystallized in n-hexane to obtain yellow-brown crystals of monovinylazobenzene (4.36 g, yield 91.5%). Then weigh monovinylazobenzene (2.38g, 0.01mol), photoinitiator benzoin diethyl ether (DMPA, 0.308g, 0.0012mol) and thioglycerol (1.44g, 0.012mol, 90%) and dissolve in 20mL methanol In the process, freezing-evacuating-filling with nitrogen, repeated three times and then placed under ultraviolet light at room temperature to react for 2h. After the reaction, the product was reverse-precipitated in 300 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
| loss modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com