Method for synthesizing ferulic acid through enzyme method
A technology of enzymatic synthesis and ferulic acid, which is applied in the directions of transferase, introduction of foreign genetic material using a carrier, recombinant DNA technology, etc., can solve the problems of high cost, low yield and long reaction time, and achieves mild reaction conditions and transformation. The effect of high efficiency and low conversion cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
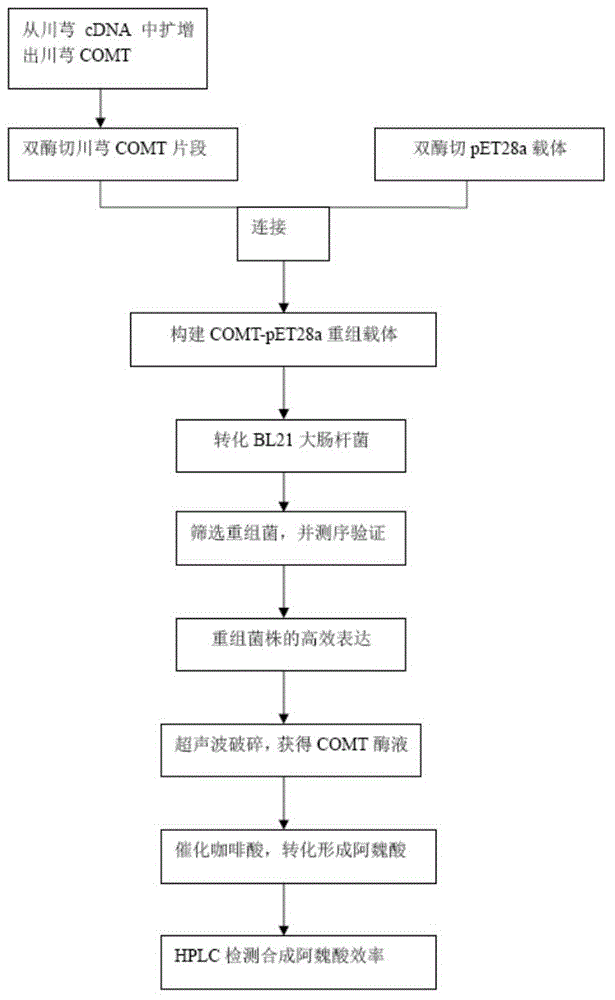
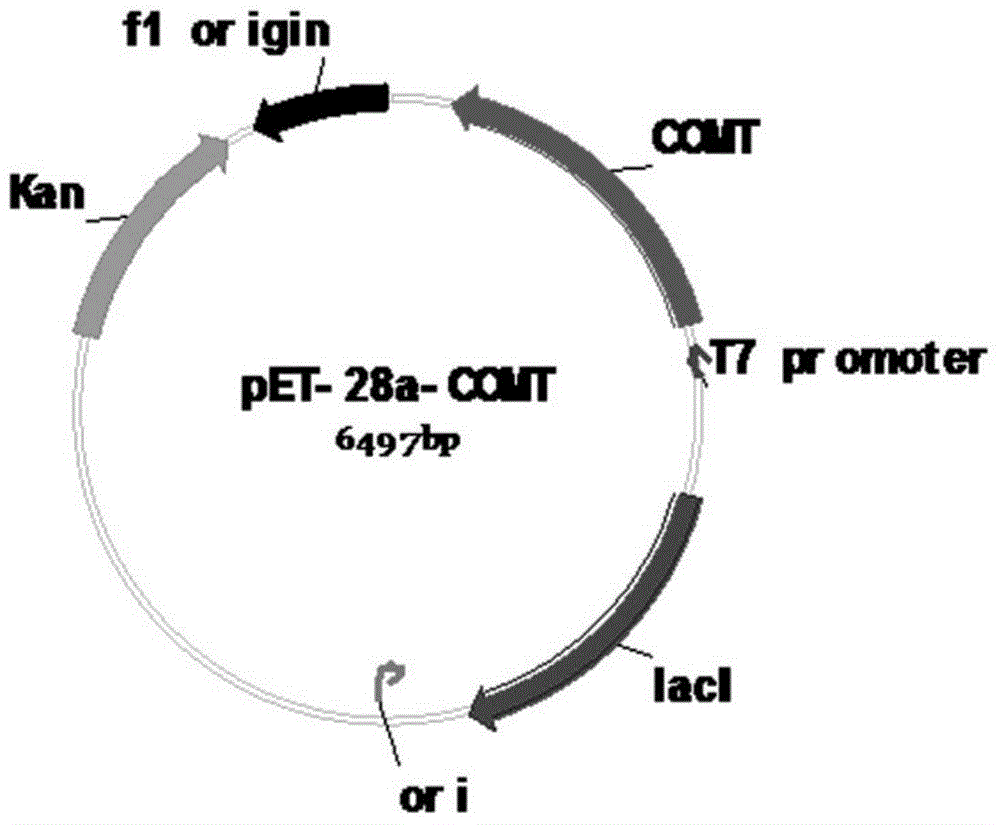
[0027] Embodiment 1: Construction of pET28a-COMT recombinant plasmid:
[0028] 1. The pET28a plasmid is a Novagen product, with a size of 5369bp, containing a strong T7 promoter, a kanamycin resistance gene, and a lactose repressor lac I gene.
[0029] 2. Design two expression primers (A1: CGCGGATCCATGAATACGGAGCTGATCCCACC; A2: CGGAATTCACATTAAGCAGATGCCAGACACCC) containing the complete reading frame of Chuanxiong COMT and BamH1 in the forward direction and EcoR1 in the reverse direction. Ligusticum chuanxiong COMT fragment containing two restriction sites of BamH1 and EcoR1 at the end. The PCR system is: Primer star mix (Bao Biological Company) 25ul, cDNA 2ul, primer 1 2ul, primer 2 2ul, double distilled water to make up 50ul. The PCR system was 94°C for 5min, 94°C for 1min, 66-68°C for 1min, 72°C for 2min, 35 cycles, 72°C for 10min, 4°C∞.
[0030] 3. After the gel was recovered, the amplified COMT fragment of Ligusticum chuanxiong and the pET28a vector were double-digested wi...
Embodiment 2
[0035] Embodiment 2: the fermentation of recombinant bacterial strain:
[0036] 1. Inoculate 1ml of the overnight cultured recombinant strain into 100ml of LB liquid medium containing 10g / L peptone, 5g / L yeast extract, 10g / L NaCl, 50mg / L kanamycin, in a 500ml Erlenmeyer flask at 37°C, Cultivate at 200 rpm for 2-4 hours to obtain seed liquid.
[0037] 2. Inoculate 5ml of seed liquid into 100ml of LB liquid medium, cultivate at 37°C and 200rpm until OD OD600=0.6. Add IPTG to make the final concentration 1mM / L, culture at 17-20°C, 200rpm for 8-10h.
Embodiment 3
[0038] Embodiment 3: the enzymatic conversion of ferulic acid
[0039] 1. Centrifuge the obtained fermentation broth at 5000g for 10min at 4°C to collect the bacterial cells.
[0040] 2. Resuspend the cells with 10ml of 20mM Tris-Hcl pH7.9 buffer, and lyse the cells by ultrasonication. The ultrasonic program was 60W, working for 9s, pausing for 6s, repeated 199 times under ice bath conditions. Centrifuge at 13000g at 4°C to remove cell debris. The supernatant was collected to obtain a mixed enzyme solution containing COMT of Ligusticum chuanxiong.
[0041] 3. Reaction system: 500uM caffeic acid, 1mM SAM, 100mM MgCl2, 1mM DTT, 100mM potassium phosphate buffer 100ml, 1-5ml mixed enzyme solution.
[0042] 4.37 ℃ stand for 6 ~ 8h. Add 5-10ml of concentrated HCl to terminate the reaction.
[0043] 5. Extract 3 times with ethyl acetate to obtain ferulic acid crude extract.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com