Probenecid sustained-release tablet and preparation method
A technology of probenecid and sustained-release tablets, which is applied in the field of medicine, can solve the problems of reduced curative effect, inconvenient administration, and poor patient compliance, and achieve the effects of simple formula, fast drug application, and increased compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
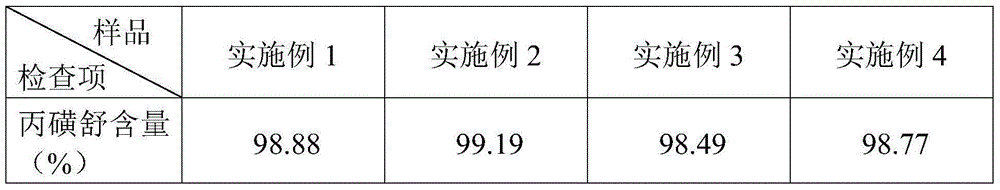
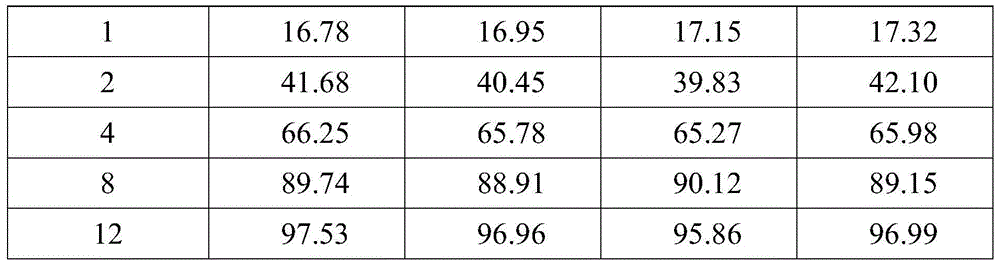
[0033] A probenecid sustained-release tablet, the components and parts by weight of the sustained-release tablet include: 45 parts of probenecid, 32 parts of sodium bicarbonate, 23 parts of sucrose pills, 4 parts of ethyl cellulose, and 0.4 part of castor oil , 0.2 parts of hypromellose, 12 parts of compressible starch, 0.8 parts of magnesium stearate, 17 parts of dry starch, and 3 parts of hydroxypropyl methylcellulose.
[0034] A preparation method of probenecid sustained-release tablets, the specific steps comprising:
[0035] 1) Material preparation: prepare materials according to the components and parts by weight of a probenecid sustained-release tablet, wherein sodium bicarbonate is pulverized through a 100-mesh sieve, and fillers, lubricants, and disintegrants are pulverized through a 80-mesh sieve;
[0036] 2) Preparation of the drug-containing pellet core: adopt the drug-applying process at the bottom of the fluidized bed, dissolve probenecid with 95% alcohol to prep...
Embodiment 2
[0042] A probenecid sustained-release tablet, the components and parts by weight of the sustained-release tablet include: 40 parts of probenecid, 30 parts of sodium bicarbonate, 20 parts of microcrystalline cellulose pellets, 3 parts of polyethylene glycol, glycerin 0.3 parts of ester, 0.1 parts of polyacrylic resin, 10 parts of microcrystalline cellulose, 0.5 parts of micropowder silica gel, 15 parts of dry starch, and 2 parts of hydroxypropyl cellulose.
[0043] A preparation method of probenecid sustained-release tablets, the specific steps comprising:
[0044]1) Material preparation: prepare materials according to the components and parts by weight of a probenecid sustained-release tablet, wherein sodium bicarbonate is pulverized through a 100-mesh sieve, and fillers, lubricants, and disintegrants are pulverized through a 80-mesh sieve;
[0045] 2) Preparation of the drug-containing pellet core: adopt the drug-applying process at the bottom of the fluidized bed, dissolve p...
Embodiment 3
[0051] A probenecid sustained-release tablet, the components and parts by weight of the sustained-release tablet include: 50 parts of probenecid, 35 parts of sodium bicarbonate, 35 parts of starch pellets, 5 parts of polyethylene, 0.6 part of succinate, 0.3 parts of polyethylene glycol, 15 parts of microcrystalline cellulose, 1.0 parts of talcum powder, 30 parts of sodium carboxymethyl starch, and 5 parts of hydroxypropyl cellulose.
[0052] A preparation method of probenecid sustained-release tablets, the specific steps comprising:
[0053] 1) Material preparation: prepare materials according to the components and parts by weight of a probenecid sustained-release tablet, wherein sodium bicarbonate is pulverized through a 100-mesh sieve, and fillers, lubricants, and disintegrants are pulverized through a 80-mesh sieve;
[0054] 2) Preparation of the drug-containing pellet core: adopt the drug-applying process at the bottom of the fluidized bed, dissolve probenecid with 98% alc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com