Bortezomib synthesis intermediate preparation method
A technology for bortezomib and intermediates, which is applied in the field of bortezomib synthesis intermediates, and can solve the problems of high content of deboronation by-products, low catalytic activity, immature process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1 Preparation of Icy.BF4
[0078] Add paraformaldehyde (12.6g), toluene (400ml), and cyclohexylamine (39.6g) into a 1000ml reaction bottle, react at room temperature for 1.5 hours, raise the temperature to 40°C, and add another portion of cyclohexylamine (39.6g) , then dropwise add tetrafluoroboric acid (40% HBF4, 88g) aqueous solution, add about 0.5 hours, stir for 10 minutes, cool down to 0°C, add glyoxal (58g), stir at 0-5°C for 2 hours, heat up to 20°C ℃, stirred at 20-25℃ for 3 hours, a large amount of solid precipitated out, cooled to 0-5℃ and stirred for 2 hours, filtered, washed the solid twice with water and twice with ether, drained, and air-dried indoors to obtain 82g of crude Icy.BF4.
[0079] Add crude Icy.BF4 (82g), dichloromethane (500ml), and ethyl acetate (500ml) into a 2000ml reaction flask, raise the temperature to reflux to dissolve the solid material, filter out the insoluble matter, and stir the filtrate at room temperature for 1 hour, 0-5 ...
Embodiment 2
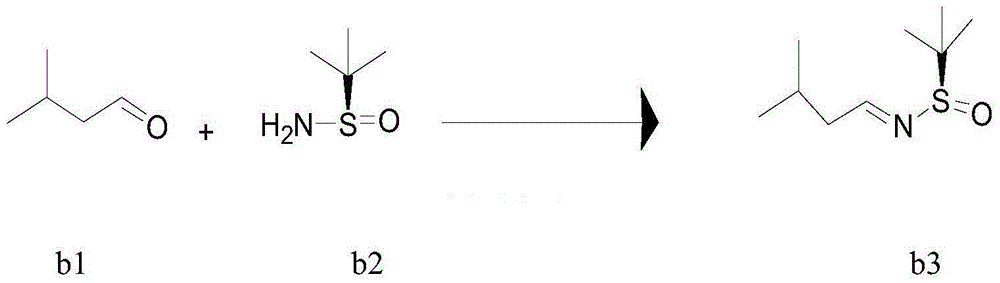
[0080] Example 2 Preparation of (R)-1-N-(tert-butylsulfinyl)-3-methylbutylimine (b3)
[0081] In a 50L reactor equipped with a reflux condenser, sequentially add (R)-tert-butylsulfinamide (b2) (0.93Kg), dichloromethane (14.9Kg), isovaleraldehyde (b1) (1.02Kg) , anhydrous magnesium sulfate (5Kg), PPTS (0.094Kg), turn on the stirring water bath and raise the temperature to reflux, the temperature of the water bath is controlled at 47-50°C, the reflux reaction is 6 hours HPLC monitoring b2 disappears, drop to room temperature and filter, wash with dichloromethane Dehydrating agent, combined with dichloromethane solution, washed twice with water, once with saturated brine, dried over anhydrous sodium sulfate for 3 hours, filtered, washed with dichloromethane, combined with dichloromethane solution, spin-dried at 40°C under reduced pressure , and then pumped under reduced pressure with a diaphragm pump for 3 hours. Obtain b3 (1.24Kg), yield 90%. This intermediate was directly use...
Embodiment 3
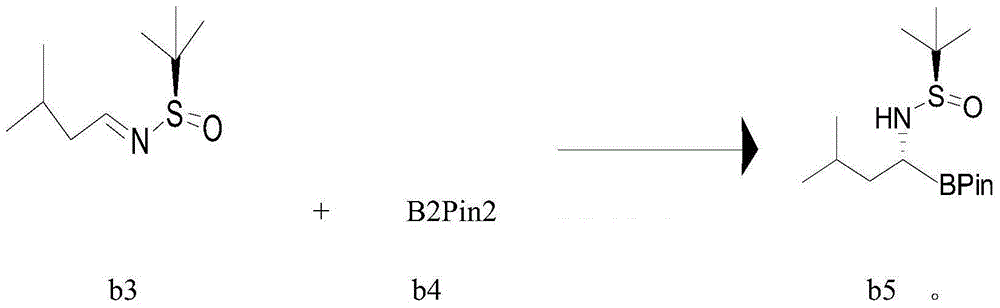
[0082] Example 3 Preparation of hydrochloride (I) of (R)-1-amino-3-methylbutylboronic acid pinacol ester
[0083] Add Icy.BF4 (0.062Kg), tetrahydrofuran (6.4Kg), potassium tert-butoxide (0.048Kg) into a 30L reactor, add CuCl (0.0192Kg), b4 (1.176Kg) and stir for 2 hours under the protection of argon , add b3 (0.72Kg) and methanol (122g), react at 20-25°C for 3 hours, TLC monitors the disappearance of raw material b3, absorb the reaction solution into a 50L separator, add ethyl acetate (16.2Kg) to dilute, and use pure Washed with water three times, dried overnight with anhydrous sodium sulfate (7.5Kg), filtered the next day, and the filtrate was concentrated to dryness at 34°C under reduced pressure to obtain a yellow oil b5 (1.07Kg), which was directly used in the next step without purification.
[0084] Dissolve b5 prepared in the previous step with dioxane (9.6Kg) and methanol (3.2Kg), transfer it to a 30L reactor, adjust the temperature to 20°C, add HCl dioxane solution (4M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com