Catalyst for electroless plating, metallic coating film produced using same, and method for producing said metallic coating film
A manufacturing method and catalyst technology, which are applied in physical/chemical process catalysts, chemical instruments and methods, liquid chemical plating, etc., can solve problems such as complicated processes, increased process costs, poor adhesion, etc., and achieve less risk of price changes, The effect of reducing process cost and reducing the number of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0096] Hereinafter, although an Example is given and this invention is demonstrated in detail, this invention is not limited to these. In addition, unless otherwise indicated, "part" and "%" are a mass basis.
[0097] The types of analysis equipment used in the present invention are as follows.
[0098] 1 H-NMR: Manufactured by JAPAN ELECTRON OPTICS LABORATORY CO.,LTD, AL300, 300Hz
[0099] TEM observation: manufactured by JAPAN ELECTRON OPTICS LABORATORY CO.,LTD, JEM-2200FS
[0100] Conductivity: HORIBA, Ltd. manufacture, B-173
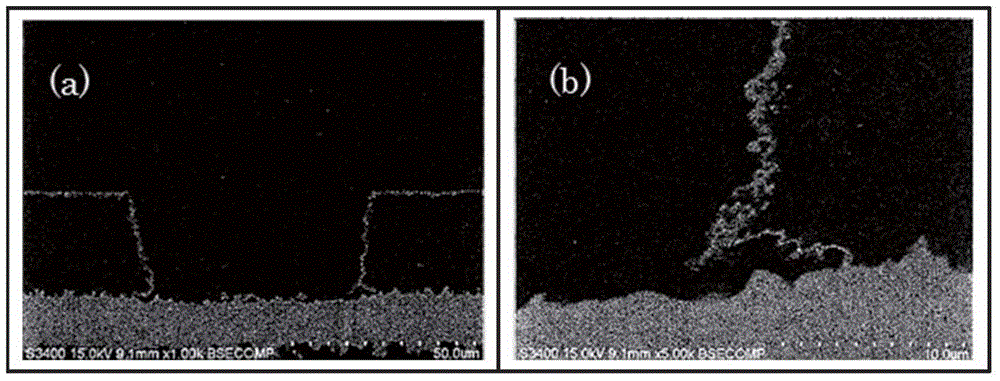
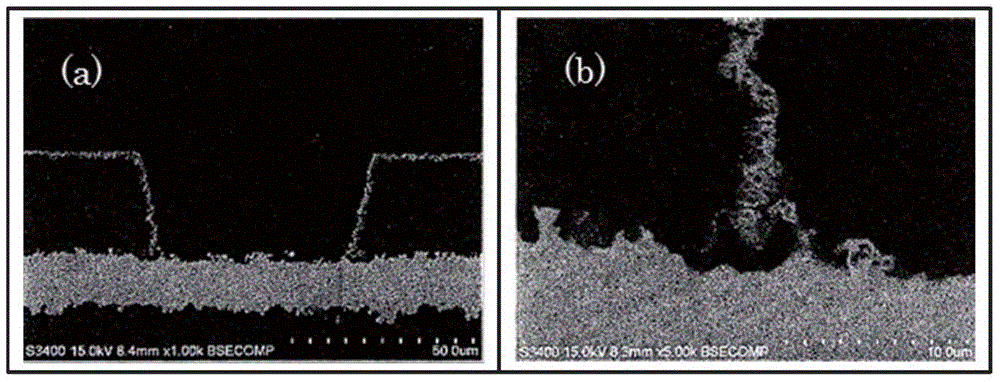
[0101] SEM observation: S-3400 manufactured by HITACHI HIGH-TECHNOLOGIES CORPORATION
[0102] TG-DTA measurement: manufactured by SII NANOTECHNOLOGY INC., TG / DTA6300
[0103] Plasma absorption spectrum: manufactured by Hitachi, Ltd., UV-3500
[0104] Dynamic light scattering particle size measurement device: manufactured by OTSUKA ELECTRONICS Co., LTD, FPAR-1000
[0105] Surface resistivity measurement: manufactured by Mitsubishi Chemical Corpor...
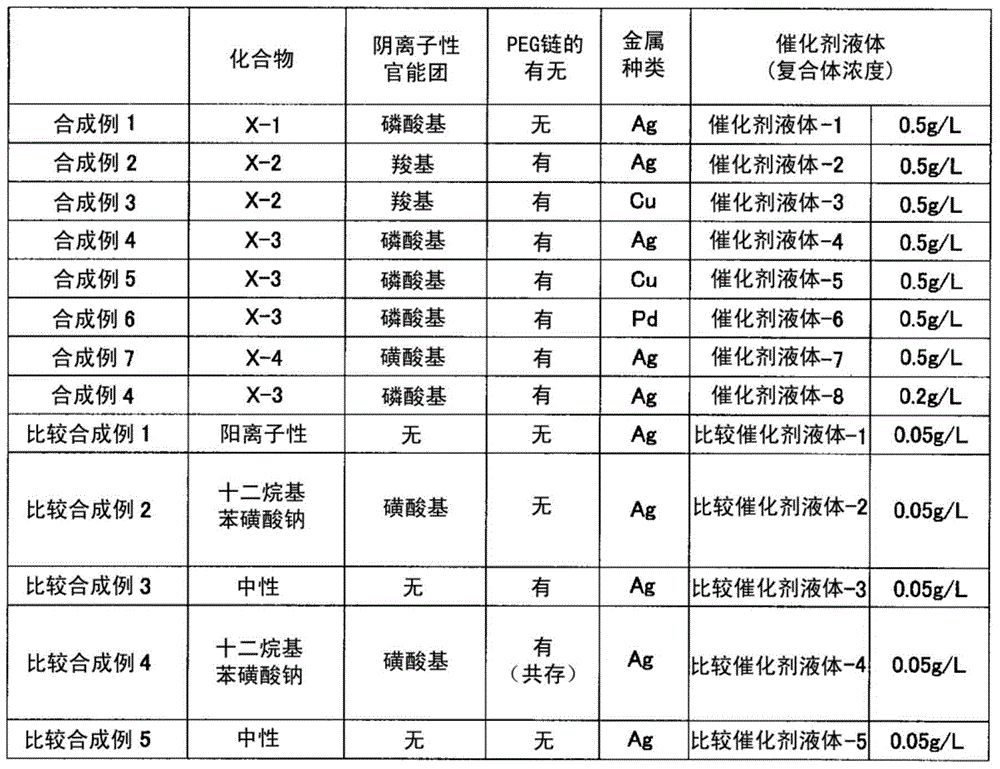
Synthetic example 1
[0109]
[0110] (Synthesis of compound (X-1) having a phosphoric acid group)
[0111] Under a nitrogen atmosphere, 210 g of ethanol and 174 g of 2-butanone were added to the reaction container, and heated to 75° C. while stirring. To this, a mixed solution obtained by dissolving 600 g of Light Ester P-1M (manufactured by KYOEISHA CHEMICAL Co., LTD.) in a mixed solvent of 90 g of ethanol and 90 g of 2-butanone was added dropwise over 3.5 hours, and dropped simultaneously over 4.5 hours. A solution obtained by dissolving 3 g of a polymerization initiator "V-59" and 18 g of a chain transfer agent (methyl 3-mercaptopropionate) in 30 g of 2-butanone was added. After 21 hours from the start of the reaction, heating was stopped, and after air-cooling to room temperature, 300 g of distilled water was added. Remove the solvent by distillation under reduced pressure with a rotary evaporator, add 100 g of distilled water to remove by distillation under reduced pressure again, and filt...
Synthetic example 2
[0116]
[0117] (Synthesis of compound (X-2) having a carboxyl group)
[0118] 70 parts of methyl ethyl ketone (hereinafter referred to as MEK) was kept at 80°C in a nitrogen stream, and 10 parts of methacrylic acid, 10 parts of benzyl methacrylate, BLEMMER PME-1000 (manufactured by NOF CORPORATION) were added dropwise over 2 hours while stirring. ) 80 parts, 2 parts of thioglycolic acid, 80 parts of MEK, and 4 parts of a polymerization initiator (“Perbutyl (registered trademark) O”, manufactured by NOF CORPORATION). After completion|finish of dripping, 2 parts of "Perbutyl (registered trademark) O" was added, and it stirred at 80 degreeC for 22 hours more. Water was added to the obtained reaction mixture to remove the solvent under reduced pressure, and then the amount of non-volatile components was adjusted with water. Thus, an aqueous solution (33% of nonvolatile content) of a compound (X-2) having a carboxyl group as an anionic functional group was obtained. The resin ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com