Synthesis and purpose of glycyrrhetinic acid derivative
A technology of glycyrrhetinic acid and derivatives, which is applied in the field of preparation of glycyrrhetinic acid derivatives, and can solve the problems of further research on liver targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1. compound synthesis experiment
[0030] 1.1 Experimental materials
[0031] 1.1.1 Main experimental instruments
[0032]XT4A binocular micro melting point apparatus, temperature uncorrected; RE-52A rotary evaporator; CCA-20 circulating water pump; CL-2 magnetic stirrer; ZF-1 ultraviolet analyzer; KQ-250DE numerical control Ultrasonic cleaner; Agilent 1100LC-MSD-Trap-SL mass spectrometer; Bruker 400 nuclear magnetic resonance spectrometer and avanceIII 500 nuclear magnetic resonance spectrometer, the solvent is DMSO-d 6 or CDCl 3 , TMS is the internal standard.
[0033] 1.1.2 Main reagents and materials
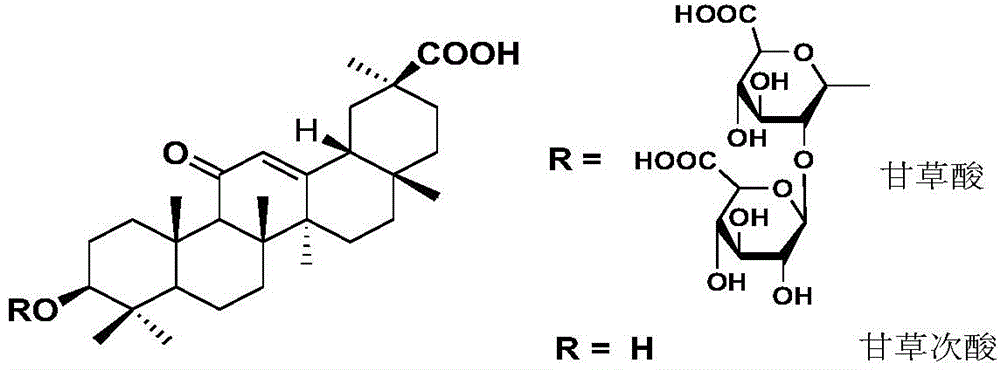
[0034] Glycyrrhetinic acid (97% 18β-glycyrrhetinic acid), norcantharidin, tetrahydrofuran (anhydrous), triethylamine (anhydrous), acetic anhydride (anhydrous), N-methylmorpholine, oxalyl chloride, Anhydrous sodium carbonate, DCC (dicyclohexylcarbodiimide), DMAP (4-dimethylaminopyridine), DMF (N,N-dimethylformamide), zinc powder, 4A molecular sieve, VLC...
Embodiment 2
[0062] Embodiment .2 compound pharmacological experiment
[0063] 2.1 Main reagents and materials
[0064] Human liver cancer cell line HepG2 was purchased from Shanghai Cell Bank Center, Chinese Academy of Medical Sciences, DMEM medium, fetal bovine serum (Hyclone, American Hyclone Company), trypsin (Huamei Bioengineering Company), dimethyl sulfoxide (Sigma Company) ), phosphate buffered solution (PBS), 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide (MTT), etc.
[0065] 2.2 Experimental operation
[0066] 2.2.1 Solution preparation
[0067] ①MTT solution (5g / L)
[0068] Weigh 250mg of MTT, put it into a small beaker, add 50mL of PBS (0.01mol / L, pH7.4) and stir on an electromagnetic stirrer for 30min, filter and sterilize with a 0.22μm microporous membrane, and dispense under aseptic conditions. Store at 4°C. Valid for two weeks.
[0069] ②10% fetal bovine serum DMEM culture medium
[0070] Aspirate 90mL DMEM culture solution, add 10mL fetal bovine serum, mi...
Embodiment 3
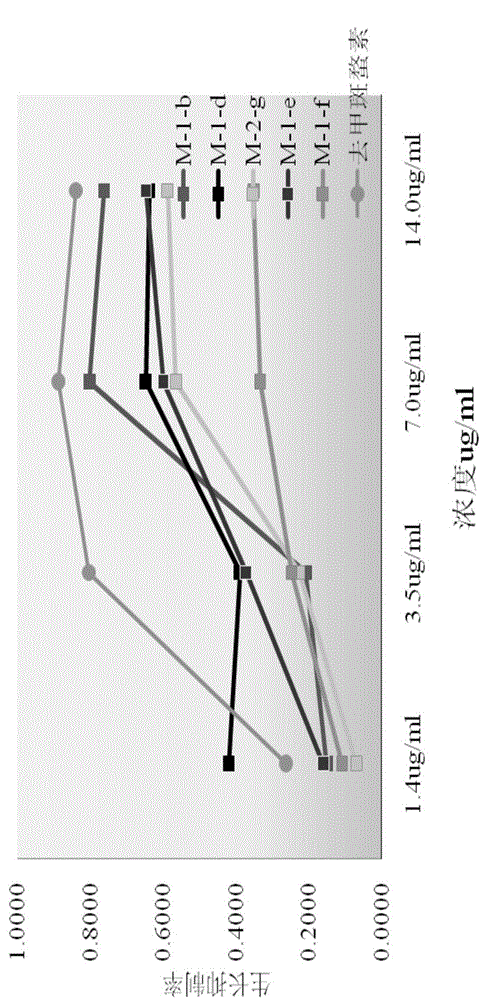
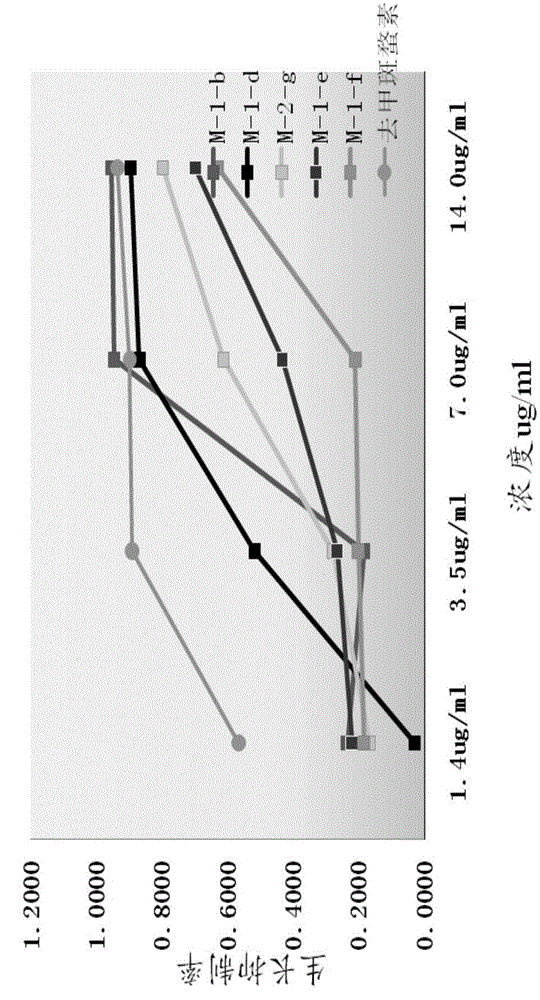
[0084] Embodiment 3: compound synthesis and pharmacological experiment result
[0085] Synthesized 13 target compounds that have not been reported in the literature, by proton nuclear magnetic resonance spectrum ( 1 H-NMR), carbon nuclear magnetic resonance spectrum ( 13 C-NMR), mass spectrometry (MS) confirmed the structural formula ( Figure 8 ). The obtained target compounds are easily soluble in tetrahydrofuran and ethyl acetate, soluble in methanol and ethanol, and almost insoluble in water.
[0086] 3.1 Analysis of Compound Spectrum
[0087] With target compound M-1-b (molecular formula C 39 h 56 o 8 , Molecular weight 652.40, structural formula see Figure 9 ) as an example to analyze its related spectral data.
[0088] 3.1.1 Proton NMR spectrum 1 H-NMR:
[0089] 1 H-NMR (400MHz, CDCl 3 )δ: 5.69 (1H, s, H-12), 4.89-5.05 (3H, m, 3CH), 4.52-4.54 (1H, m, CH), 4.13-4.15 (1H, m, H-3), 3.01 -3.08(3H,m,COOCH 3 ),2.37(1H,s,H-9),2.08(1H,m,H-18),1.86(2H,m,CH 2 ),1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com