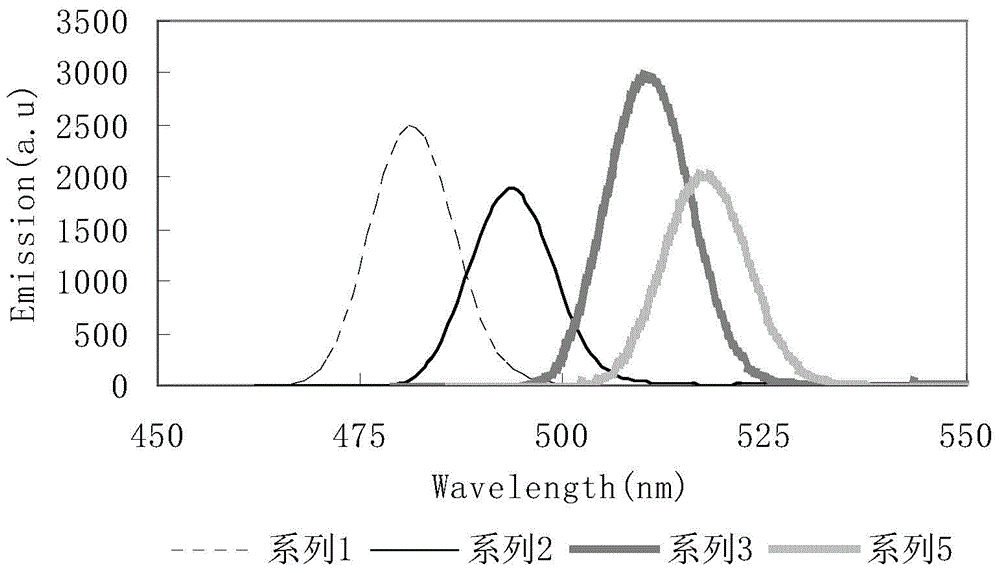
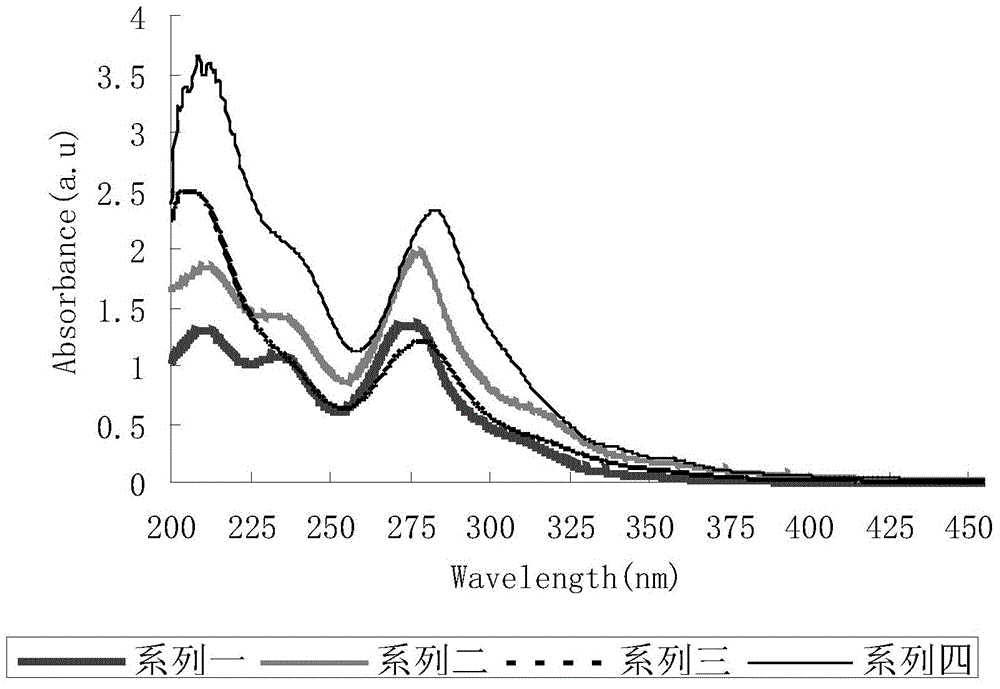
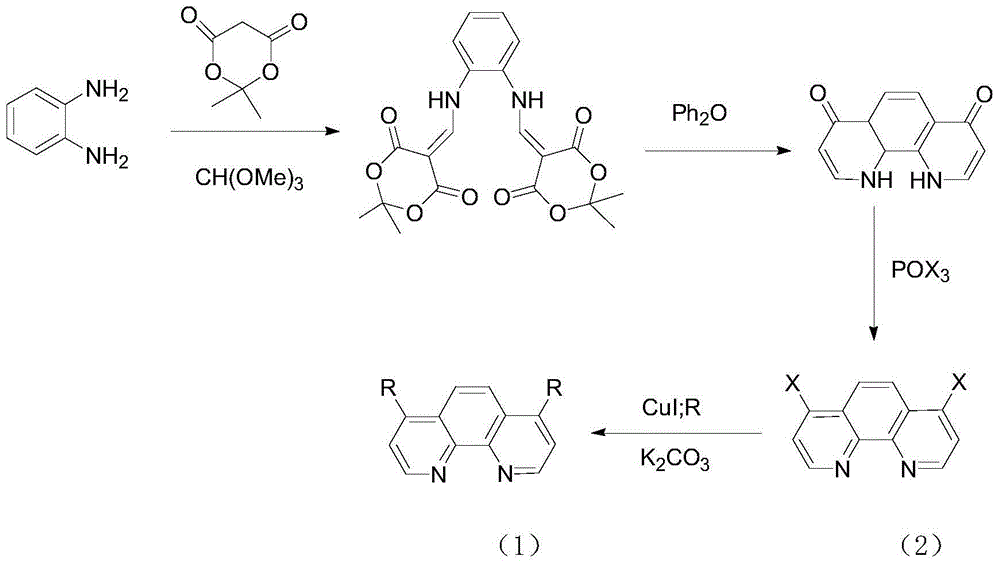
4,7 substituted-1,10-phenanthroline derivative and preparation method thereof
A technology of o-phenanthroline and its derivatives, applied in 4 fields, can solve the problems of low luminous efficiency, complex preparation process, high energy consumption, etc., and achieve remarkable technological progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of Compound K1
[0025]
[0026] Get McBurney's acid (5.05g, 7.3mmol), 50ml of trimethyl orthoformate in a 100ml round-bottomed flask, mix them and reflux at 106°C for two hours while stirring. After the reflux was completed, the solution was yellow-red. After the solution was cooled, the weighed (1.625 g, 1.8 mmol) o-phenylenediamine was added. And continue to reflux for one hour. After the reflux was completed, the solution was evaporated to dryness with a rotary evaporator, and a yellow solid was precipitated until there was no liquid in the bottle. The solid was washed three times with absolute ethanol (50 ml), and suction filtered to obtain a light yellow powder. Afterwards, it was recrystallized with ethanol to obtain 4.5 g of white needle crystal product M1. Yield: 78%, mp>209°C, Rf=0.56 (ethyl acetate), 1H NMR (DMSO) δ1.66 (s, 12H), 7.42 (d, 4H), 7.61 (d, 4H), 8.26 ( d, 2H), 11.32(d, 2H).
[0027] Take M1 (0.416g, 1mmol) and reflux ...
Embodiment 2
[0030] Example 2 Preparation of Compound K2
[0031]
[0032]Get M3 (0.0338g, 0.1mmol), benzimidazole (0.0296g, 0.25mmol), salt of wormwood (0.069g, 0.5mmol), 2mlDMF joins in the 50ml round-bottomed flask, gets an appropriate amount of copper iodide as catalyst, in React at 135°C for 24h. During the experiment, under nitrogen protection, the reaction solution was extracted with 100ml of dichloromethane and washed with water 6 times, DMF was removed, dried by rotary evaporation, and column chromatography gave 0.04568g of a light yellow powder product. Yield: 85%, Mass Spectrum: m / z=413.3[M]+; NMR: 1H NMR (CDCl3) δ7.21 (d, 2H) 7.34 (t, 2H) 7, 42 (t, 2H) 7.67 (s , 2H) 7.83 (d, 2H) 7.98 (d, 2H) 8.24 (s, 2H) 9.49 (d, 2H).
Embodiment 3
[0033] Example 3 Preparation of Compound K3
[0034]
[0035] Take M3 (0.12g, 0.4mmol), pyrazole (0.055g, 0.8mmol), potassium carbonate (0.11g, 1mmol), and 4ml of DMF into a 50ml round bottom flask, take an appropriate amount of copper iodide as a catalyst, Under reaction 24h. During the experiment, under the protection of nitrogen, the reaction solution was extracted with 100ml of dichloromethane and washed 6 times with water, DMF was removed, dried by rotary evaporation, and 0.056g of a light yellow powder product was obtained by column chromatography. Yield: 55%, Mass Spectrum: m / z=313.3[M]+; NMR: 1H NMR (CDCl3) δ6.67 (s, 2H) 7.76 (d, 2H) 7.96 (d, 2H) 8.02 (d, 2H) )8.32(s,2H)9.32(d,2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com