A kind of thioether functionalized metal carboxylic acid molecular cage and its synthesis method
A metal carboxylic acid and functionalization technology, which is applied in chemical instruments and methods, copper organic compounds, and other chemical processes, can solve the problems of not being able to use metal ions, etc., and achieve unique aesthetic value, unique structure, and rich valence states Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
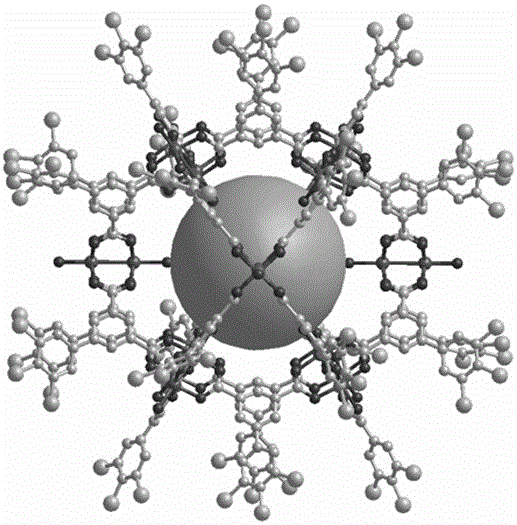
[0045] A kind of thioether functionalized metal carboxylic acid molecular cage of the present embodiment has a chemical formula of C 408 h 384 o 120 S 72 Cu 24 , and the chemical structural formula shown in formula I:
[0046]
[0047] Formula I.
[0048] Among them, in the chemical structural formula represented by formula I, the copper atom adopts a hexacoordinated octahedral configuration, and every two copper atoms form a two-copper cluster, and the bond length of the Cu-Cu bond in the two-copper cluster is 2.734(1) Å, Each dicopper cluster is coordinated with ten O atoms, among which, eight of the ten O atoms come from the carboxylic acid functional groups on the four ligands, and the other two of the ten O atoms come from the Two coordinated water molecules, and the two coordinated water molecules are respectively located at the axial position of the Cu–Cu bond in the two copper clusters.
[0049] among them, see figure 1 , the thioether functionalized metal c...
Embodiment 2
[0053] A kind of synthetic method of thioether functionalized metal carboxylic acid molecular cage of the present embodiment, it comprises the following steps:
[0054] Step 1, the preparation of intermediate product 3',4',5'-tri(methylthio)biphenyl-3,5-dimethyl ester
[0055] Put 5-bromo-1,2,3-trithiomethylbenzene, 3,5-dimethoxycarbonylphenylboronic acid, potassium carbonate and catalyst tetraphenylphosphine palladium into two-necked round-bottomed flasks, and Add the deoxygenated organic solvent ethylene glycol dimethyl ether into the bottom flask, and then under the protection of nitrogen, pass nitrogen gas into the two-necked round bottom flask, and then react at 85°C for 60h to obtain the reaction solution. After cooling, pour the reaction solution into water (the water in this embodiment is distilled water), add dichloromethane for extraction, then use a rotary evaporator to remove the organic solvent, and the remaining substances after removing the organic solvent pass ...
Embodiment 3
[0064] A kind of synthetic method of thioether functionalized metal carboxylic acid molecular cage of the present embodiment, it comprises the following steps:
[0065] Step 1, the preparation of intermediate product 3',4',5'-tri(methylthio)biphenyl-3,5-dimethyl ester
[0066] Put 5-bromo-1,2,3-trithiomethylbenzene, 3,5-dimethoxycarbonylphenylboronic acid, sodium carbonate and catalyst tetraphenylphosphine palladium in two-necked round-bottomed flasks, and Add deoxygenated organic solvent toluene into the bottom flask, and then pass nitrogen gas into the two-necked round-bottomed flask under the protection of nitrogen, and then react at 90°C for 48 hours to obtain a reaction solution. After the reaction solution is cooled, the reaction Liquid is poured into water (the water in this embodiment is distilled water), and dichloromethane is added for extraction, and then the organic solvent is removed by a rotary evaporator, and the remaining substances are separated and purified by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com