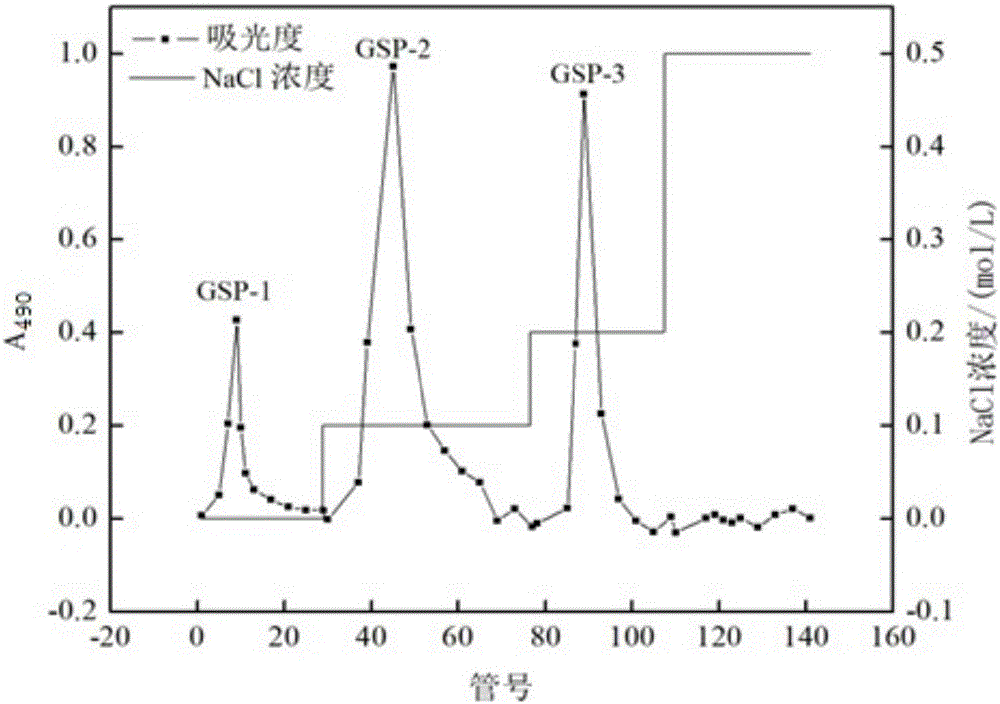
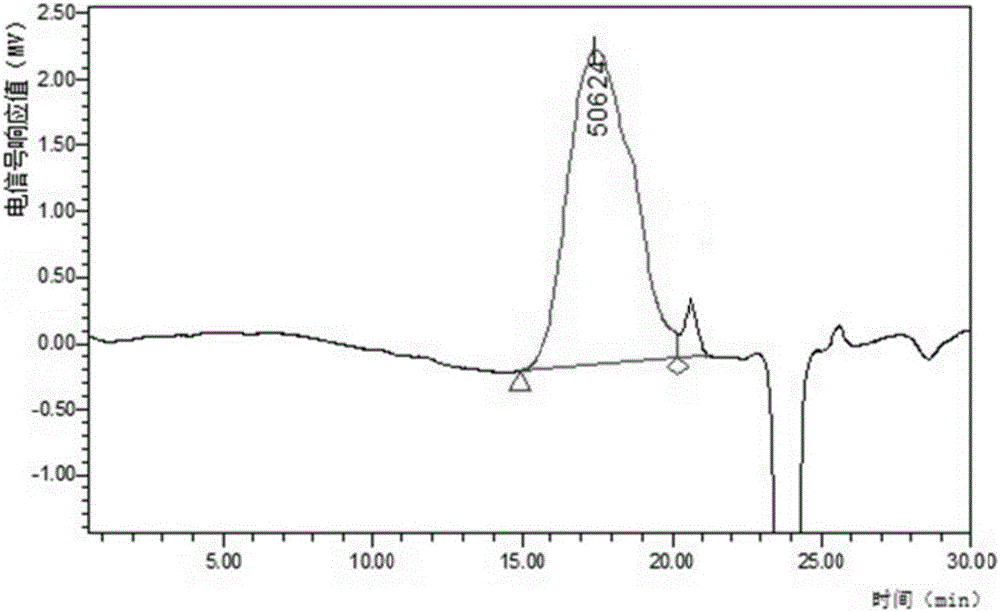
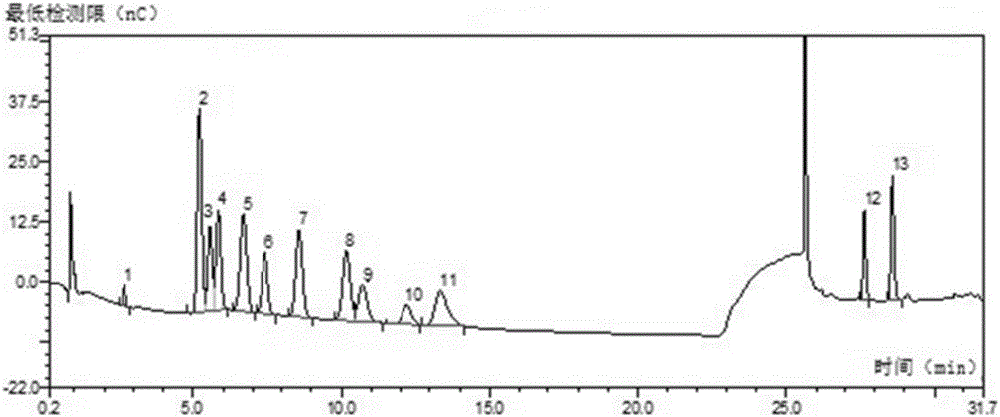
Gentian acidity uniform polysaccharide and purification method and application thereof
A purification method and technology of gentian are applied in the field of gentian acidic homogeneous polysaccharide and its purification, which can solve the problems of few reports on the separation and purification of gentian polysaccharide, the determination of molecular weight of components and the unreported anticoagulant activity, etc. The effect of developing application prospects, simple and feasible operation, and avoiding potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]A gentian acidic homogeneous polysaccharide, comprising the following monosaccharides and their molar percentages: 1.1% fucose, 11.5% rhamnose, 22.5% arabinose, 17.9% galactose, 3.3% glucose, xylose 3.1%, mannose 0.7%, galacturonic acid 39.9%. The specific preparation method is as follows:
[0036] polysaccharide extraction
[0037] After cleaning the root of gentian, dry it in an oven at 60°C, then pulverize it with a pulverizer, and pass through an 80-mesh sieve to obtain gentian powder. The mass-volume ratio of gentian powder and distilled water to the feed liquid is 1:25g / mL was leached at 90°C for 3 hours, and after suction filtration, the filtrate was obtained, and the filtrate was vacuum concentrated to 20% of the original volume at 55°C (the purpose was to reduce the amount of ethanol and subsequent centrifugation times) to obtain a concentrate , slowly add 4 times the volume of absolute ethanol and keep stirring, and place it in a refrigerator at 4°C for over...
Embodiment 2
[0062] Preparation of anticoagulant health product—gentian drink
[0063] In parts by weight, the ratio of water to gentian coarse powder is 1 to 15:100, the extraction temperature is 75 to 95°C, and the time is 5 to 30 minutes. ℃, placed for 24h to clarify, and 0.5μm fine filtration to obtain clarified gentian juice. 0.04-0.2% gentian acid homogeneous polysaccharide GSP-3, 3-6% sucrose, 0.03-0.07% ascorbic acid and other auxiliary materials are firstly dissolved in some warm water and filtered. Mix it with the finely filtered gentian juice, sterilize at 135°C for 5-7s, and bottle it to obtain an anticoagulant health-care gentian drink.
Embodiment 3
[0065] Preparation of anticoagulant drugs
[0066] Get 4g gentian acid homogeneous polysaccharide GSP-3, 500g microcrystalline cellulose, 450g dextrin, 75g low-substituted hypromellose, 75% ethanol 75mL, magnesium stearate 5g, press 2000 by wet granulation tableting method piece.
[0067] Granules: take 4g gentian acid homogeneous polysaccharide GSP-3, 1400g dextrin, 100g powdered sugar, 50g tartaric acid, 50% ethanol in appropriate amount, use wet granulation to sieve, mix soft materials, extrude into granules, and dry at 60°C , packaged after whole grains, 2-5g per bag.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com