1,2-diazine derivatives and their preparation and use
A derivative, C1-C4 technology, applied in the field of 1,2-diazine derivatives and their preparations, can solve the problems of oral delivery of hydrophilic macromolecular drugs, unreachable, poor stability, etc., to achieve convenient treatment Disease mode, stability-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
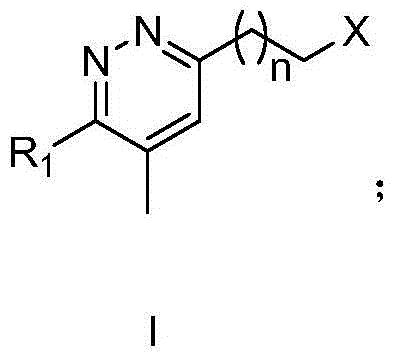
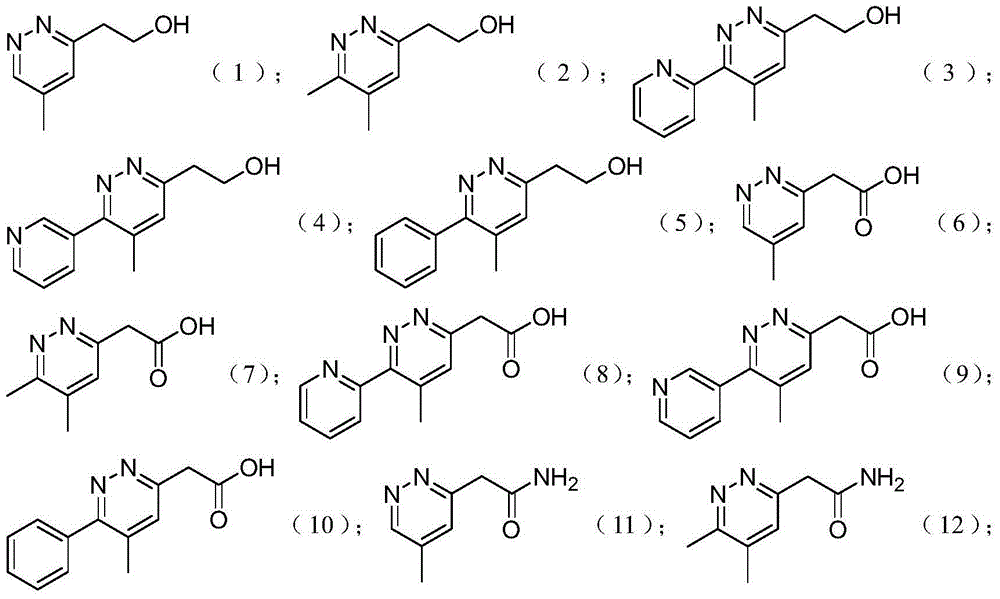
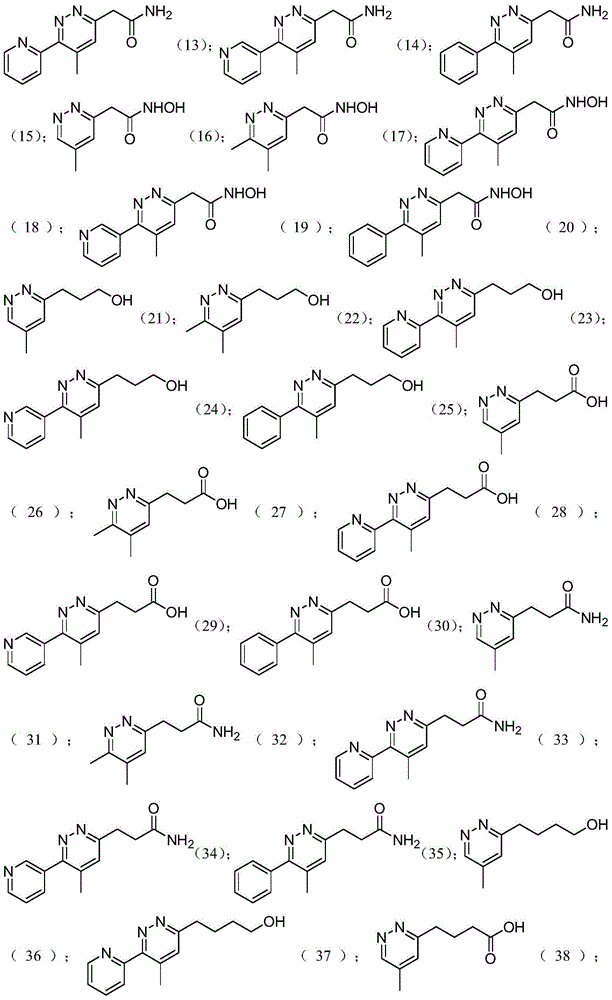
[0032] Embodiment 1: Preparation of 1,2-diazine derivatives
[0033] Method 1: Add hydrazine (10.0 equivalents) to a flask mixed with formamidine acetate (4.0 equivalents), sulfur (1.0 equivalents), and ω-cyano compound (1.0 equivalents), and stir the mixture vigorously at 25°C for 20 hours , was added to the acetic acid solution, stirred for 20 minutes and filtered, and the filtrate was collected. An aqueous solution of sodium nitrite (3.0 equivalents) was added to the collected acetic acid solution, and then the solution was cooled at 0°C for 1 hour. Under reduced pressure, acetic acid was removed with a rotary evaporator, and the residue was washed with acetonitrile, filtered, and then recrystallized to obtain pure tetrazole compound (0.5 eq, 50%). The structure of the compound was confirmed by H NMR or mass spectrometry.
[0034]
[0035]Method 2: Add hydrazine (10.0 equivalent) to a flask mixed with cyano compound (4.0 equivalent), sulfur (1.0 equivalent), and ω-cyan...
Embodiment 2
[0047] Example 2: Oral administration of heparin to rats
[0048] Heparin is an anticoagulant, which is a polymer composed of two polysaccharides connected alternately, and has anticoagulant effects both in vivo and in vitro. Clinically, it is mainly used for thromboembolic diseases, myocardial infarction, cardiovascular surgery, cardiac catheterization, extracorporeal circulation, hemodialysis, etc.
[0049] Preparation of heparin oral administration preparation: take 1,2-diazine derivative and dissolve in 30% propylene glycol aqueous solution according to the amount of compound and heparin shown in the table, and adjust the pH value to 7.4 with aqueous sodium hydroxide solution, then stir Allow 20 minutes for the solution to become a homogeneous oral dosing mixture.
[0050] Male rats with a weight of 200-250 grams were fasted for 24 hours, and ketamine (44 mg / kg) was injected intramuscularly into the rats 15 minutes before administration, and then a latex tube with a diame...
Embodiment 3
[0073] Embodiment 3: Oral administration of salmon calcitonin to rats
[0074] Salmon calcitonin inhibits the activity of osteoclasts; inhibits the dissolution of bone salts and prevents the release of calcium in bones; improves bone density and effectively relieves pain symptoms; reduces the risk of fractures; reduces blood calcium.
[0075] Preparation of oral dosage forms of salmon calcitonin: Dissolve the 1,2-diazine derivatives listed in Table 3 in an equivalent amount of sodium hydroxide aqueous solution, so that the pH of the solution is about 7-8. Calcitonin from salmon was weighed and dissolved in an appropriate amount of citric acid aqueous solution (0.08 equivalents), then the two solutions were mixed and fully stirred to form a homogeneous solution for later use.
[0076] Male rats with a weight of 200-250 grams were fasted for 24 hours, and ketamine (44 mg / kg) / chlorpromazine (1.5 mg / kg) was intramuscularly injected into the rats 15 minutes before administration, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com