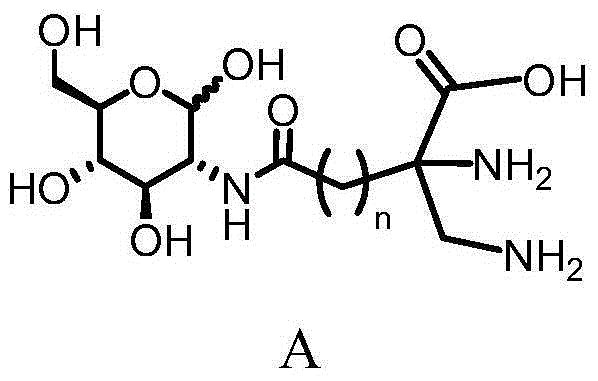
Glucosamine derivative ligand compound and preparing method, tricarbonyl Tc-99m labeled complex and preparing method and application of tricarbonyl Tc-99m labeled complex
A ligand compound, glucosamine technology, applied in the field of clinical nuclear medicine, can solve problems such as limited use of cyclotrons, short half-life, etc., and achieve stable performance, easy preparation, and simple preparation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
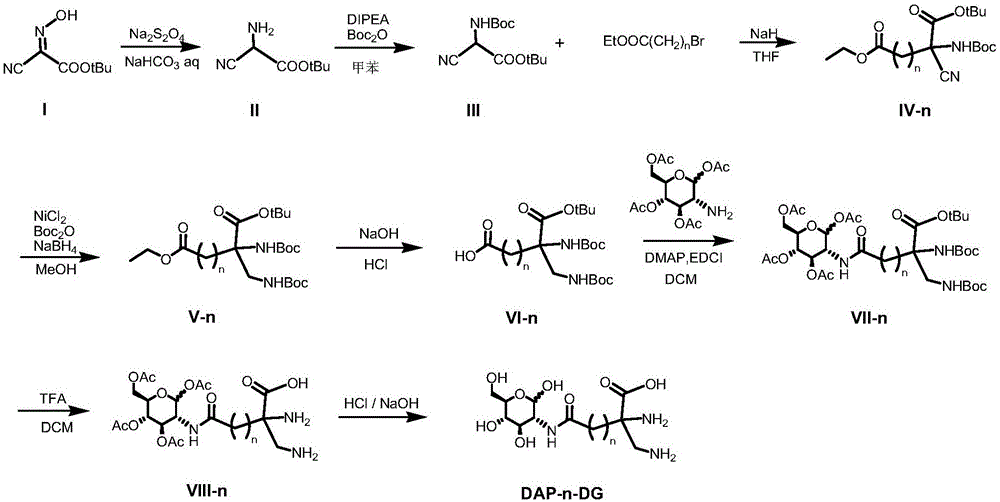
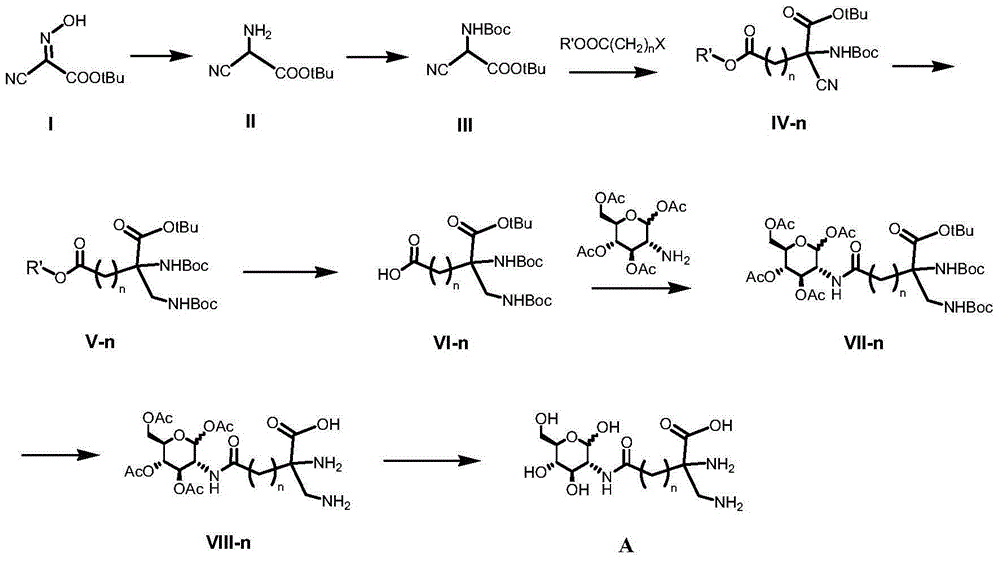
[0045] Embodiment 1 Preparation of glucosamine derived ligand compound (DAP-n-DG)
[0046] Such as figure 1 shown, including the following steps:
[0047] (1) Synthesis of tert-butyl 2-amino-2-cyanoacetate (II)
[0048] Add 11.98 g of tert-butyl 2-oximino-2-cyanoacetate (I) into 88 mL of deionized water, add 80 mL of saturated aqueous sodium bicarbonate solution prepared in advance, and react under stirring for about 40 min. The reaction bottle was placed in a 40°C oil bath, and a total of 33 g of sodium hydrosulfite was added in portions. The reaction is about 9h. Add 15g of sodium chloride, fully dissolve and cool to room temperature. The mixture was repeatedly extracted with dichloromethane (50 mL each time), the organic phase extract was dried over anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation to obtain 2.10 g of brown oil (II), with a yield of 19%.
[0049] (2) Synthesis of 2.2-(N-tert-butoxycarbonylamino)-2-tert-butyl cyanoacetate (I...
Embodiment 2 3
[0077] Example 2 Preparation of Tricarbonyl Technetium-99m Labeled Complexes
[0078] Weigh 5.5 mg of sodium borohydride, 20 mg of sodium potassium tartrate, and 4 mg of sodium carbonate into penicillin vials, cover with a rubber stopper and then seal with an aluminum cap, extract air with an aspirating needle, and inject CO gas into it for 10-20 min. will be used from medical 99 Mo- 99m Sodium pertechnetate Na[ 99m TCO 4 ] Eluent 2mL (about 2mCi·mL -1 ), added into the reaction flask, shaken to dissolve, and then reacted for 30 minutes under the condition of heating in a metal bath at 75°C to obtain the tricarbonyl technetium-99m intermediate. Add 1mol·L -1 hydrochloric acid to adjust the pH to 7.
[0079] Dissolve 10mg DAP-2-DG in 0.5mL 0.5mol·L -1 In the pH7.0 phosphate buffer solution, add tricarbonyl technetium-99m intermediate solution. React at 95°C for 15 minutes. get 99m Tc(CO) 3 -DAP-2-DG.
[0080] Through thin layer chromatography and HPLC identification...
Embodiment 3 3
[0081] Example 3 Preparation of Tricarbonyl Technetium-99m Labeled Complexes
[0082] Weigh 5.5 mg of sodium borohydride, 20 mg of sodium potassium tartrate, and 4 mg of sodium carbonate into penicillin vials, cover with a rubber stopper and then seal with an aluminum cap, extract air with an aspirating needle, and inject CO gas into it for 10-20 min. will be used from medical 99 Mo- 99m Sodium pertechnetate Na[ 99m TCO 4 ] Eluent 2mL (about 2mCi·mL -1 ), added into the reaction flask, shaken to dissolve, and then reacted for 30 minutes under the condition of heating in a metal bath at 75°C to obtain the tricarbonyl technetium-99m intermediate. Add 1mol·L -1 hydrochloric acid to adjust the pH to 7.
[0083] Dissolve 2mg DAP-3-DG in 0.5mL 0.5mol·L -1 In pH7.0 phosphate buffer solution, add tricarbonyl technetium-99m intermediate solution. React at 95°C for 15 minutes. get 99m Tc(CO) 3 -DAP-3-DG.
[0084] Through thin layer chromatography and HPLC identification, it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com