Mold for manufacturing aneurysm model and method
An aneurysm and mold technology, applied in the field of medical animal model establishment, can solve the problems of blood vessel damage, complicated operation, and many human interventions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
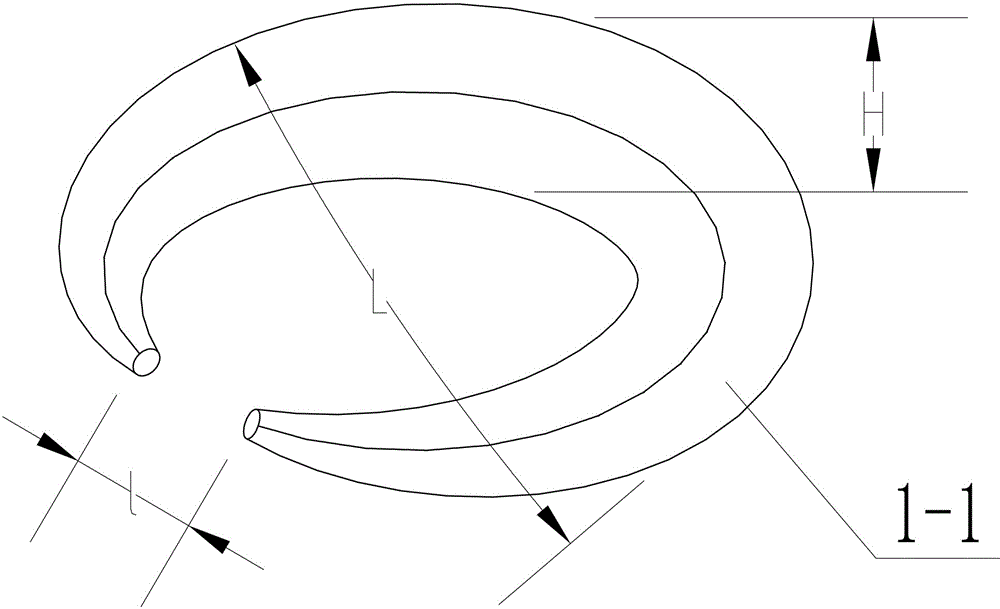
[0032] Such as figure 1 As shown, the mold used in this embodiment is an open circular ring, and the material of the mold is polytetrafluoroethylene; polytetrafluoroethylene is a polymer compound formed by polymerization of tetrafluoroethylene, which has excellent chemical stability and corrosion resistance. Sex, sealing, high lubricity and non-stickiness, electrical insulation and good anti-aging endurance, it is physiologically inert, non-toxic, and has no adverse reactions when implanted as artificial blood vessels and organs for a long time. The mold height H, that is, the thickness of the ring is 1 cm, the middle diameter, that is, the width of the widest part L of the mold, that is, the outer diameter of the ring is 3 cm, and the height of the two ends is slightly lower than the middle, and the height of the two ends is 0.8 cm. The opening width l is 0.3 cm.
[0033] The establishment method of the aneurysm animal model of the present embodiment, its specific embodiment...
Embodiment 2
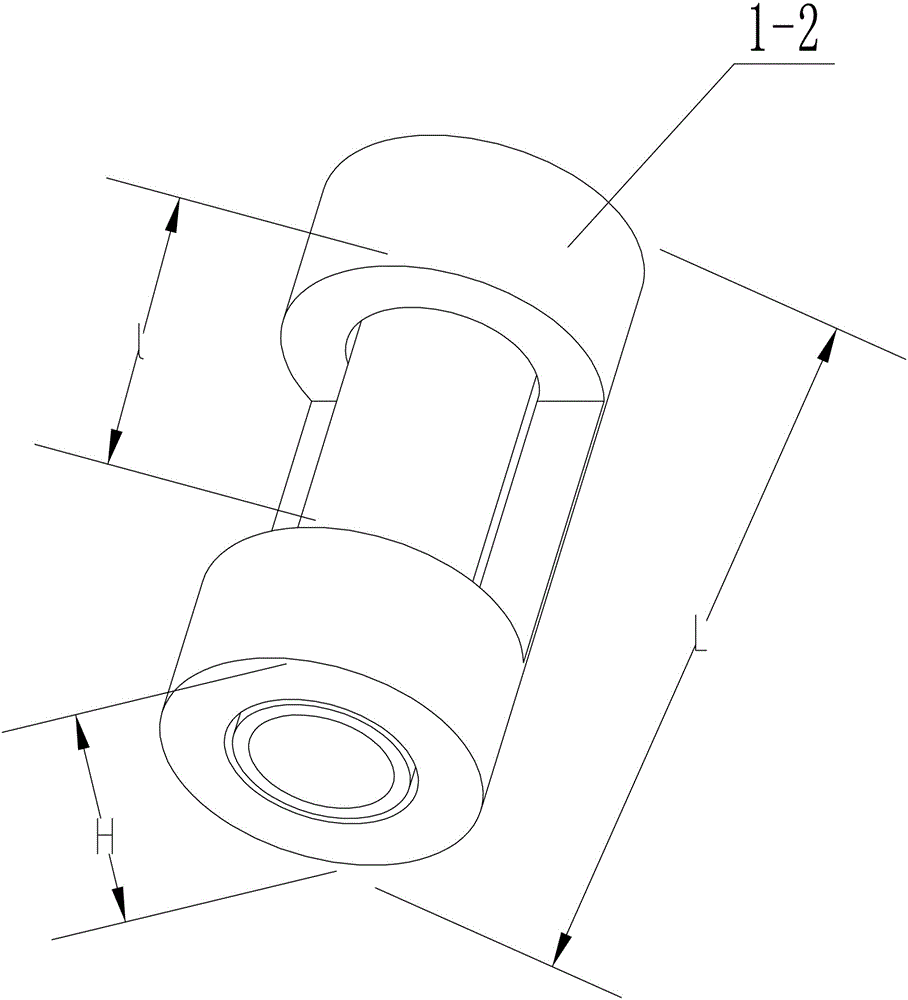
[0038] In this embodiment, the selected mold is concave, such as figure 2 As shown, the material of the mold is biological titanium alloy, which has strong corrosion resistance, good compatibility with human cell tissue, no allergic reaction, high strength and low elastic modulus; the height H of the mold is 1.1cm , the distance between the middle opening of the mold, that is, the width l of the mold opening, is 0.45cm, and the distance between the two ends, that is, the length of the widest part L of the mold, is 0.9cm.
[0039] The establishment method of the aneurysm animal model of the present embodiment, its specific embodiment comprises the following steps:
[0040] Step 1. Randomly select 40 healthy clean rats, male or female, weighing 395-405 g, and randomly divide them into two groups, 20 rats in the control group and 20 rats in the experimental group. After fasting for 12 hours, the rats in the experimental group were anesthetized. The anesthetic was 2% pentobarbit...
experiment example 1
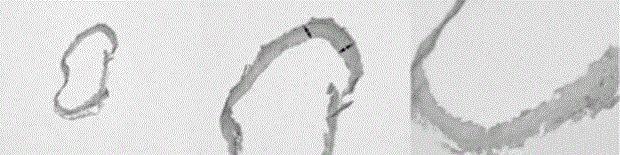
[0047] Experimental example 1: Pathological section and HE (hematoxylin-eosin staining) staining of vessel wall
[0048] In the 4th, 6th, and 8th months after the operation, select 6 rats of the control group in Example 1, and randomly select 6 rats in the experimental group to complete the modeling in Example 1, and perform the following operations on the rats: use Rats were anesthetized with 2% pentobarbital at a dosage of 2mL / Kg, the skin was prepared, disinfected with iodine, the skin and muscles were gradually incised using surgical methods, the abdominal cavity of the rat was opened, the abdominal aorta was separated, and the mold pad was removed The curved section of the high abdominal aorta; the curved section of the abdominal aorta was washed with normal saline, and then immediately fixed in neutral formalin fixative for 5-10 hours to form an abdominal aorta specimen. Embed the above abdominal aorta specimen, the steps are as follows:
[0049] 1) Dehydration: The volum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com