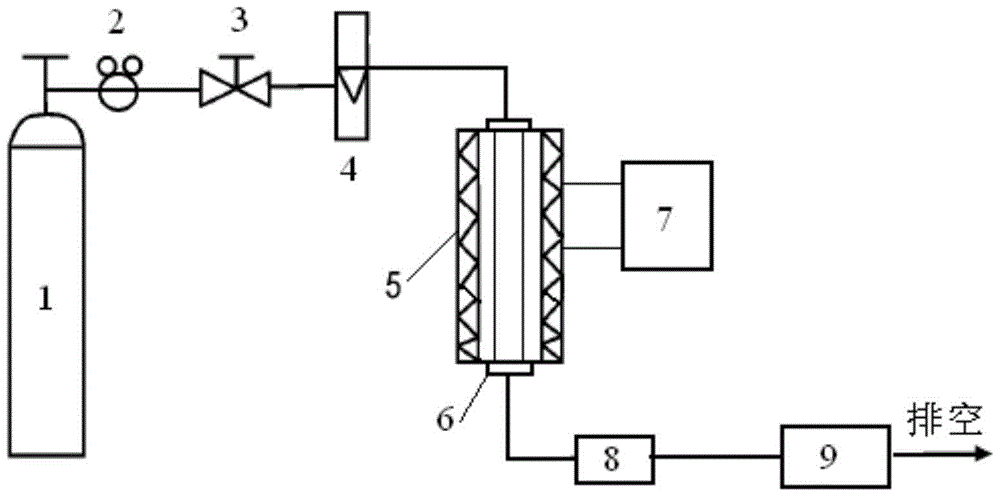
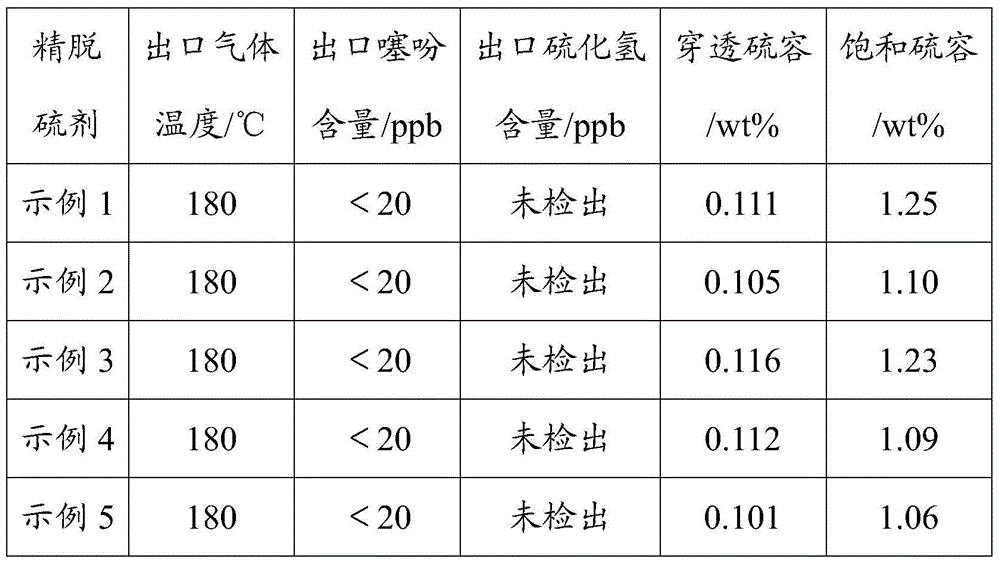
A fine desulfurizer and preparation method thereof
A technology of fine desulfurization and carrier, applied in the field of fine desulfurizer and its preparation, can solve the problems of catalyst poisoning, corrosion of industrial pipelines and equipment, and reduced product quality, and achieves good mechanical strength, large specific surface area, and improved desulfurization effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] Specifically, the preparation method of the refined desulfurizer of the present invention comprises the following steps:
[0036] Step a: One or both of zinc nitrate, manganese nitrate and magnesium nitrate are mixed with aluminum nitrate to prepare a mixed salt solution. Specifically, distilled water can be used to prepare the above mixed salt solution. Wherein, the ratio of the amount of aluminum nitrate to one or both of zinc nitrate, manganese nitrate and magnesium nitrate is controlled to be greater than 2 or less than 1 / 2. The fine desulfurizer prepared by adopting the above ratio can effectively enrich the pores in the desulfurizer carrier. Specifically, after high-temperature calcination, oxides containing a high proportion of aluminum or a high proportion of zinc, manganese, magnesium, etc. are formed, which can be passed through the subsequent acid. These oxides are dissolved to enrich the pores of the carrier.
[0037] Step b: using an alkaline solution as ...
example 1
[0046] Example 1: Preparation of fine desulfurizer
[0047] (1) 182g aluminum nitrate and 446g zinc nitrate are mixed and added to 3000mL distilled water to be mixed with mixed salt solution, then the mixture solution is heated to 60 ℃ and then loaded into No. 1 constant temperature storage tank;
[0048] (2) 2700 mL of Na with a mass concentration of 10% 2 CO 3 The solution was heated to 60°C and put into the No. 2 constant temperature storage tank;
[0049] (3) the solutions in the No. 1 constant temperature storage tank and the No. 2 constant temperature storage tank are added dropwise to the constant temperature reactor in parallel to carry out co-precipitation reaction, and the control reaction time is 20min, and the pH value in the reaction process is 10.2;
[0050] (4) the reaction product in step (3) is filtered, washed to obtain filter cake;
[0051] (5) drying the filter cake obtained in step (4) at 100° C. for 8h;
[0052] (6) in the intermediate product that st...
example 2
[0055] Example 2: Preparation of fine desulfurizer
[0056] (1) 562g aluminum nitrate and 148g zinc nitrate are mixed and added to 4500mL distilled water to be mixed with mixed salt solution, and then the mixture solution is heated to 65 ℃ and then loaded into No. 1 constant temperature storage tank;
[0057] (2) 3500mL mass concentration of Na is 10% 2 CO 3 The solution was heated to 65°C and put into the No. 2 constant temperature storage tank;
[0058] (3) the solution in the No. 1 constant temperature storage tank and the No. 2 constant temperature storage tank is added dropwise to the constant temperature reactor in parallel to carry out co-precipitation reaction, the control reaction time is 22min, and the pH value in the reaction process is 9.2;
[0059] (4) the reaction product in step (3) is filtered, washed to obtain filter cake;
[0060] (5) drying the filter cake obtained in step (4) at 120° C. for 8h;
[0061] (6) in the intermediate product that step (5) obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com