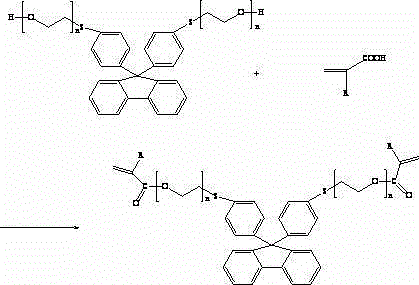
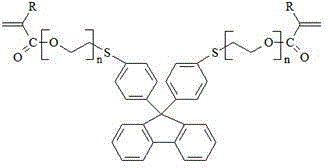
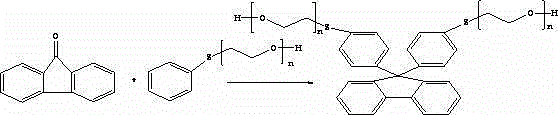
Photocuring resin containing polyethoxy bisthiophenyl fluorene structure and preparation method thereof
A technology of ethoxybisphenylthiofluorene and curing resin, which is applied in the field of structural photocurable resin containing polyethoxybisphenylthiofluorene and its preparation, can solve the problem that the refractive index cannot reach more than 1.55 and the refractive index cannot Reach 1.58, poor heat resistance and yellowing resistance, etc., achieve high refractive index, excellent self-healing performance, and good self-healing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add 11.76 g of 9.9-(4-phenylthio-2-hydroxyethoxy) bisfluorene, 4.68 g of acrylic acid, and 49.32 g of toluene to a 100 ml four-neck flask equipped with a mechanical stirrer, a condenser and a water separator. 0.43g of ethylsulfonic acid, 0.016g of p-hydroxyanisole, and 0.32g of ethyl hypophosphite were reacted at 115-120°C for 5 hours under reflux to generate 0.88-0.98g of water, and continued to reflux for 1 hour without changing the amount of water.
[0049] After the reaction, the reaction mixture was washed with 10wt% NH 4 NO 3 Solution washing once, 5wt% KOH aqueous solution alkali washing once until the pH value is about 8, then washing three times with distilled water, and finally drying with 2.5wt% anhydrous magnesium sulfate, adding a polymerization inhibitor para-hydroxyl to the dried solution Anisole 0.01g, under certain temperature conditions, the vacuum degree is 0.093~0.095MPa, and the solvent toluene is distilled off under reduced pressure to obtain a co...
Embodiment 2
[0052] Add 11.76 g of 9.9-(4-phenylthio-2-hydroxyethoxy) bisfluorene, 5.59 g of methacrylic acid, and 52.05 g of toluene to a 100 ml four-neck flask equipped with a mechanical stirrer, a condenser and a water separator g, 0.37g of trifluoromethanesulfonic acid, 0.017g of 2,6-di-tert-butyl-p-cresol, and 0.35g of ethyl phosphite. After the reaction was refluxed at 115-120°C for 3 hours, 0.93-1.03g of water was generated. Continue to reflux for 1h, and the water volume will not change.
[0053] After the reaction, the reaction mixture was washed once with 10wt% NaCl solution, once with 5wt% NaOH aqueous solution until the pH value was about 8, then washed three times with distilled water, and finally dried with 2.5wt% anhydrous magnesium sulfate. Add 0.01g of polymerization inhibitor tert-butyl-p-cresol in the solution of the above-mentioned solution, under certain temperature condition, vacuum degree is 0.093~0.095MPa, underpressure distillation removes solvent toluene, obtains ...
Embodiment 3
[0056] Add 13.61 g of 9.9-(4-phenylthio-hydroxydiethoxy) bisfluorene, 4.68 g of acrylic acid, 54.87 g of toluene, and 0.27g of methyl sulfonic acid, 0.018g of p-hydroxyanisole, and 0.36g of ethyl hypophosphite. During the reaction, nitrogen protection was always passed. After reflux reaction at 115-120°C for 4 hours, 1.05-1.15g of water was generated, and the reflux was continued for 1 hour. The amount of water no longer changes.
[0057] After the reaction finished, the reaction mixture was washed once with 10wt% NaCl solution, once with 5wt% KOH aqueous solution until the pH value was about 7, then washed three times with distilled water, and finally dried with 2.5wt% anhydrous magnesium sulfate, and then applied to Add 0.01 g of polymerization inhibitor p-hydroxyanisole to the solution after drying, and under certain temperature conditions, the vacuum degree is 0.093 ~ 0.095 MPa, and the solvent toluene is removed with a rotary evaporator to obtain a colorless and transpare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal resistance | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com