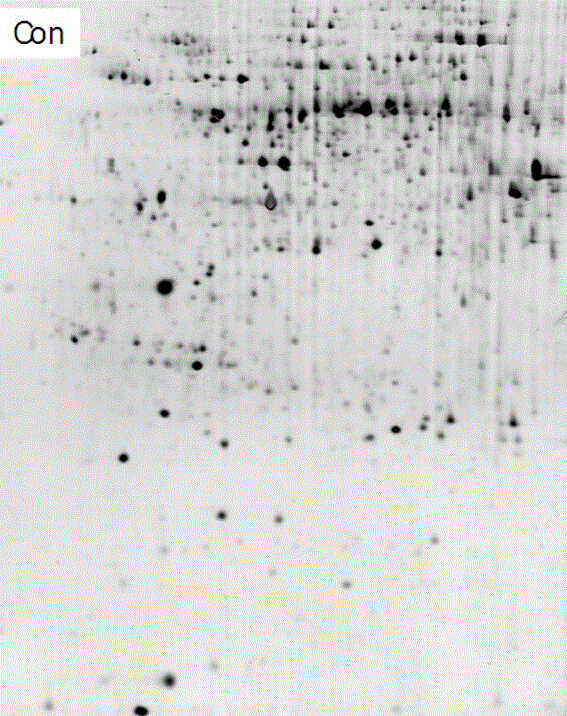
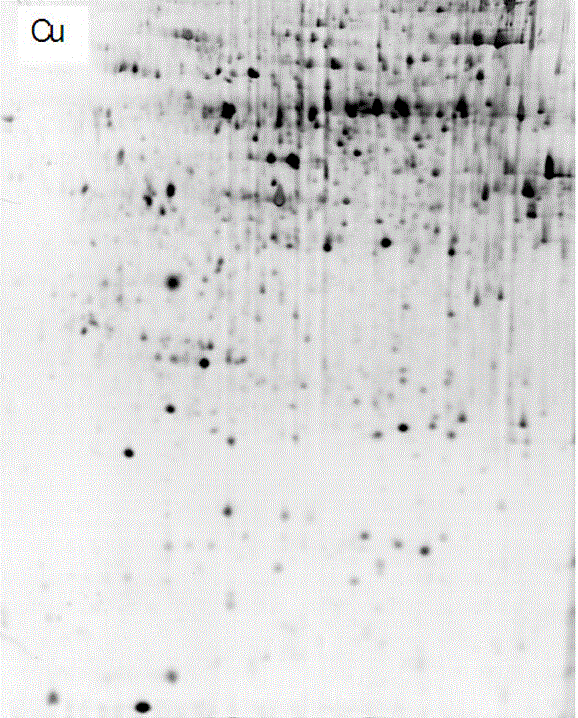
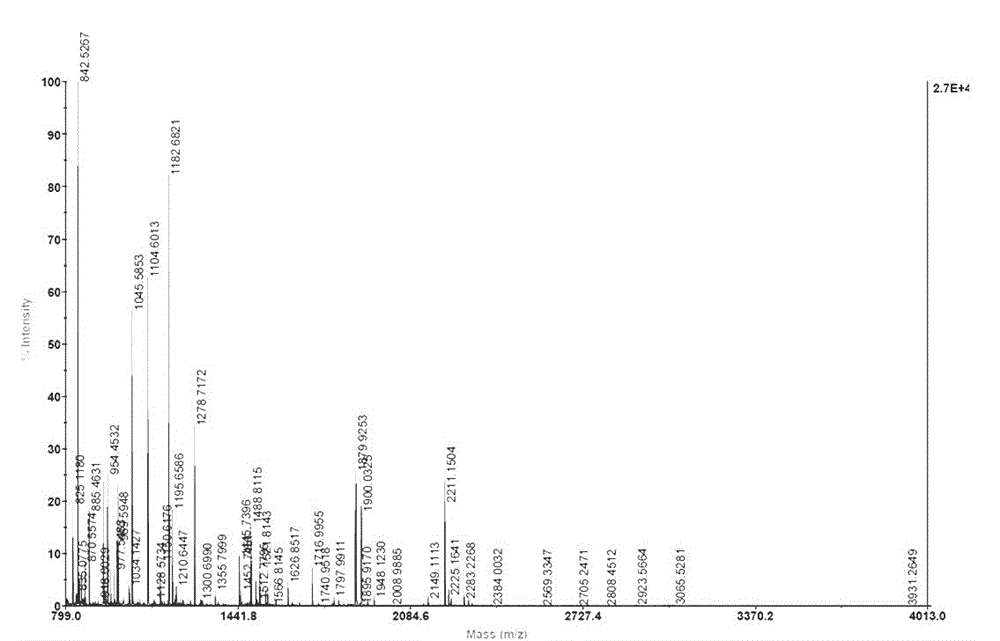
Method for separating copper proteome by using copper-chelated magnetic beads
A copper protein and magnetic bead separation technology, which is applied in the field of protein chemistry, can solve the problems of difficult separation of metalloproteome, limited use range, and low price, and achieves the effects of avoiding aggregation and precipitation, stable complexes, and simple procedures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Preparation of magnetic beads with surface chelated copper
[0037] (1) Activation of hydroxyl magnetic beads: add 5M sodium hydroxide solution to hydroxyl magnetic beads (Luoyang, Huier Nano) to make the pH value reach 12-14, and obtain fully activated magnetic beads (concentration: 10mg / ml ).
[0038] (2) Epoxidation: (1) Add dimethyl sulfoxide, epichlorohydrin and 5M sodium hydroxide solution to the obtained activated hydroxyl magnetic beads (the volume ratio of magnetic beads, epichlorohydrin and sodium hydroxide solution is 1 :2:1:4), stirred slowly at 60°C overnight and then washed to neutral to connect epoxy groups to obtain epoxidized magnetic beads.
[0039](3) Chelating-based IDA coupling: (2) Add 1 volume of IDA (10%, adjusted to pH 8 with 1M sodium hydroxide) to each ml of the obtained epoxidized magnetic beads (10mg / ml), slowly Stir for 10 hours, wash until neutral and blot dry to obtain metal chelate magnetic beads (magnetic beads-IDA).
[0040] (4) ...
Embodiment 2
[0050] A method for separating copper proteomes using copper chelated magnetic beads, comprising the following steps:
[0051] (1) Take the biological tissue or cell and grind it sufficiently with liquid nitrogen, add protein extract, mix well, and centrifuge at low temperature to obtain denatured protein solution; the solid-liquid ratio of the biological tissue or cell to the protein extract after grinding is: 1:4; the composition of the protein extract solution is: every liter of protein solution contains 50mmol / L sodium phosphate, pH7.8, 300mmol / L sodium chloride, 0.1% by mass TritonX-100, 8mol / L Urea, the balance being water;
[0052] (2) According to the volume ratio of 10 mg / ml copper chelated magnetic beads and denatured protein solution volume ratio of 1:1, mix copper chelated magnetic beads with a concentration of 10 mg / ml with the denatured protein solution obtained in (1) Mix the protein solution and incubate at 25°C-37 for 15-30min to obtain a magnetic bead protei...
Embodiment 3
[0059] A method for separating copper proteomes using copper chelated magnetic beads, comprising the following steps:
[0060] (1) After the biological tissue or cells are fully ground with liquid nitrogen, add protein extract, mix well, and centrifuge at 15000g for 30min at 4°C to obtain denatured protein solution; after grinding, biological tissue or cell and protein extract The solid-liquid ratio is: 1:4; the composition of the protein extract is: every liter of protein solution contains 50mmol / L sodium phosphate, pH7.8, 300mmol / L sodium chloride, and the mass percentage is 1% TritonX -100,8mol / L urea, the balance is water;
[0061] (2) According to the volume ratio of 10 mg / ml copper chelated magnetic beads and denatured protein solution volume ratio of 1:1, mix copper chelated magnetic beads with a concentration of 10 mg / ml with the denatured protein solution obtained in (1) Mix the protein solution and incubate at 25°C-37 for 15-30min to obtain a magnetic bead protein m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com