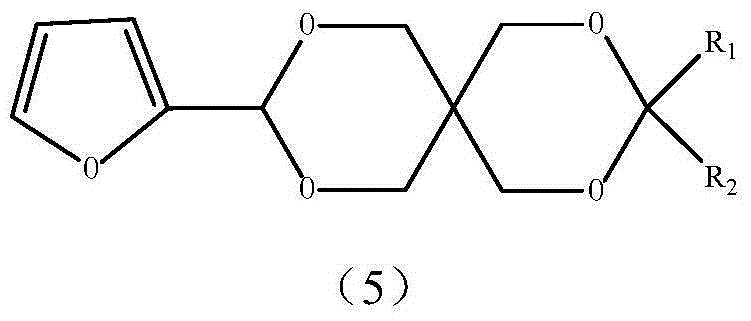
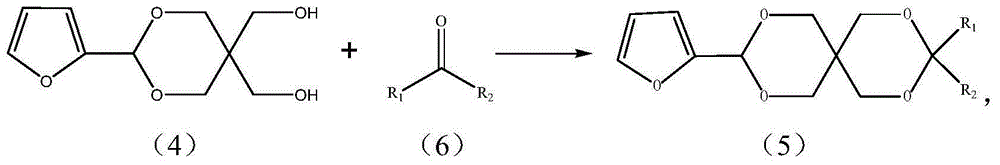
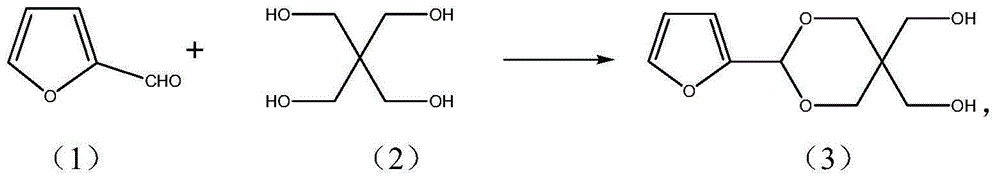Alpha-furan ring-containing pentaerythritol hetero diacetal (ketone) and preparation method thereof
A technology of pentaerythritol and furfural, which is applied in the direction of chemicals used in biological control, fat production, disinfectants, etc., can solve the problems of inability to remove, lower yield, and long reaction time, so as to shorten the reaction time and improve Excellent reaction rate and catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Put commercial activated carbon in an oven to dry (120°C, 2 hours), put it in a desiccator to cool, weigh 5.0g of activated carbon and put it into a round bottom flask, pipette 2.0mL of concentrated sulfuric acid, and insert a thermometer , control the sulfonation temperature at about 120°C, and stir the reaction for 2 hours. Add twice distilled water to stop the reaction, and wash until the filtrate is neutral. Put it in an oven and dry it at 120°C until it reaches a constant weight to obtain usable self-made sulfonated carbon.
Embodiment 2
[0038] Embodiment 2 prepares pentaerythritol monofurfural (3)
[0039]In a 50ml round bottom flask, add 1g (0.0073mol) pentaerythritol, 0.65g (0.0067mol) furfural, 15mL N, N-dimethylformamide (DMF), 6mL cyclohexane, 0.17g self-made sulfonated carbon catalyst, Upper water separator and reflux condenser, heated in an oil bath (80-90°C), stirred and refluxed for 3 hours, then cooled, filtered to remove catalyst sulfonated carbon, and added 0.2g NaHCO to the filtrate 3 Solid, fully stirred for 30 minutes, filtered off the solid, evaporated the solvent under reduced pressure, added 40 mL of ethyl acetate after cooling to dissolve the solid, washed the solution with 15 mL of water three times, separated the water layer, added 5 g of anhydrous sodium sulfate to the organic layer and dried overnight, The solvent was distilled off, and the residue was recrystallized from benzene to obtain 1.08 g of colorless needle-like crystals, with a yield of 75.3%, m.p.130.4°C; 1 HNMR (CDCl 3 ,40...
Embodiment 3
[0040] Embodiment 3 prepares the heterodiacetal (5a) of pentaerythritol and furfural and benzaldehyde
[0041] Add 1.0g (0.0047mol) of pentaerythritol monofurfural (3), 0.50g (0.0047mol) of benzaldehyde, 15mL of DMF, 6mL of cyclohexane, 0.15g of self-made catalyst sulfonated carbon into a 50mL round bottom flask, and install a water separator Heater and reflux condenser, heated in an oil bath (80-90°C), stirred and refluxed for 3 hours, then cooled, filtered to remove the catalyst sulfonated carbon, and added 0.2gNaHCO to the filtrate 3 Solid, fully stirred for 30 minutes, filtered off the solid, evaporated the solvent under reduced pressure, added 40mL of ethyl acetate after cooling to dissolve the solid, then washed 3 times with 10mL of water, separated the water layer, added 3g of anhydrous calcium chloride to the organic layer and dried overnight . The solvent was distilled off, and the residue was recrystallized from petroleum ether and ethyl acetate to obtain 0.87 g of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com