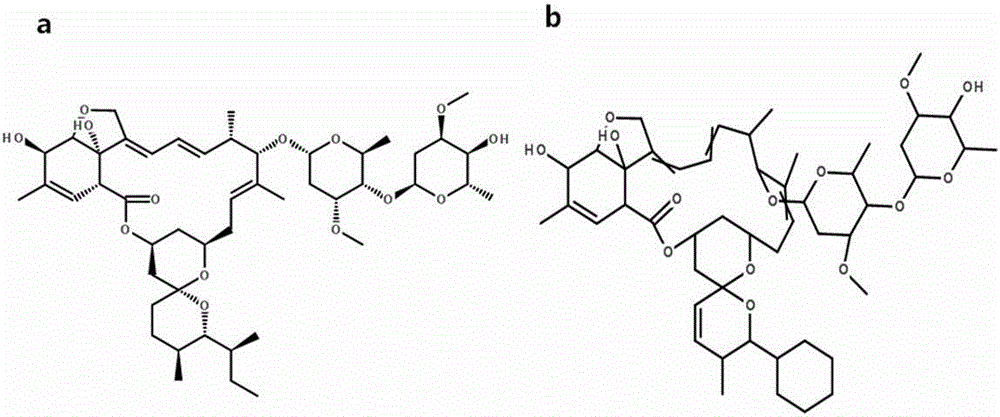
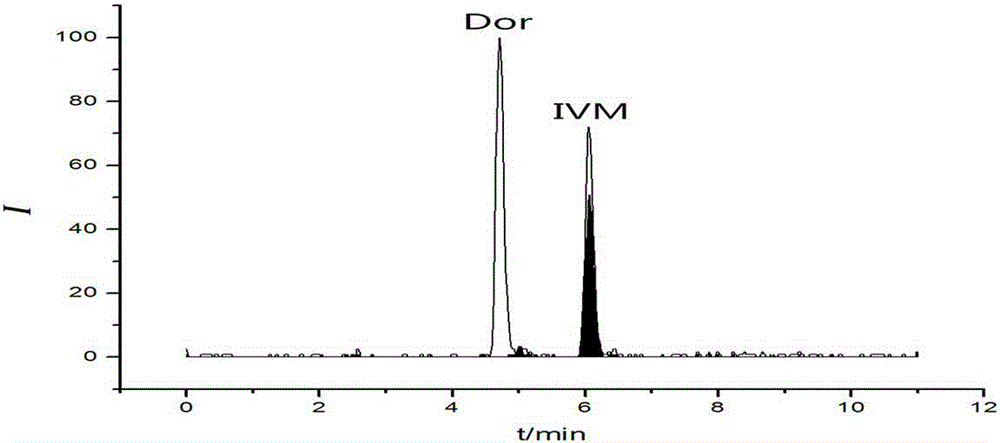
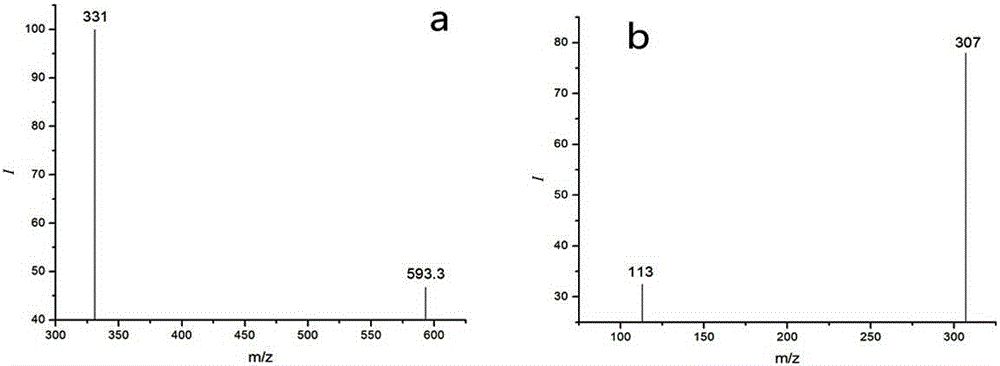
Method for detecting residual quantity of ivermectin in sheep muscle tissues by using liquid chromatograph/mass spectrometer with doramectin as internal standard substance
A muscle tissue, LC/MS technology, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of ivermectin loss, complicated and tedious operation procedures, etc., and achieves low detection limit, low coefficient of variation, and high sensitivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Effect of different ion source desolvation temperatures on the form of ion adducts
[0046] The ESI ion source is a soft ionization method, and ivermectin is usually combined with H in the ESI ion source + , Na + ,NH 4 + are combined to form quasi-molecular ion peaks, where [M+Na] + The ion structure is stable, if it is cracked by secondary mass spectrometry, it is not easy to obtain fragment ions, and the H + ,NH 4 + etc. can compete with the target molecule, thereby causing [M+Na] +The ion abundance is extremely unstable, so [M+NH 4 ] + as the parent ion.
[0047] 1. Conditions of liquid chromatography and mass spectrometry
[0048] 1. Liquid chromatography conditions
[0049] Analytical column: Agilent ZORBAX EclipsePlusC 8 Column; mobile phase A: acetonitrile, mobile phase B: 10 mmol / L ammonium acetate aqueous solution containing 0.1% formic acid (volume percentage); flow rate: 0.2 mL / min; column temperature: 25 °C; injection volume: 5 μL.
...
Embodiment 2
[0056] The standard curve assay experiment of embodiment 2 ivermectin
[0057] 1. Experimental steps
[0058] (1) Preparation of standard solution
[0059] 1. Preparation of doramectin internal standard solution
[0060] (1) Preparation of standard stock solution: Accurately weigh 10 mg of doramectin standard substance, dissolve and dilute it with acetonitrile, prepare a 100 μg / mL standard stock solution, and store it in a -20°C refrigerator in the dark.
[0061] (2) Preparation of standard solution: Accurately pipette 1 mL of doramectin standard stock solution into a 100 mL volumetric flask, dilute to the mark with acetonitrile and mix well to obtain a 1 μg / mL standard solution, store at 4°C for later use.
[0062] 2. Preparation of ivermectin standard solution
[0063] (1) Preparation of standard stock solution: Accurately weigh 10 mg of ivermectin standard substance, dissolve and dilute it with acetonitrile, prepare a 100 μg / mL standard stock solution, and store it in ...
Embodiment 3
[0083] The methodological verification of the residual determination of embodiment 3 ivermectin
[0084] By investigating method sensitivity, repeatability and recovery rate indicators, the method of the present invention is verified methodologically.
[0085] 1. Sensitivity experiment
[0086] According to embodiment 2 steps (two) to (six) process and analyze and measure the added sheep muscle sample, obtain the baseline noise of the added mark sheep muscle in the ivermectin retention time range, detect according to the calculation method of 3 times signal-to-noise ratio method Out of the limit, 10 times the signal-to-noise ratio method to calculate the quantitative limit. The test results show that the detection limit of the method of the present invention is 0.3 μg / kg, and the quantification limit is 0.5 μg / kg. The limits of ivermectin residues in sheep muscle in the United States, the European Union and my country are shown in Table 2. The detection limit and quantific...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com