Tenofovir disoproxil fumarate oral tablet and preparation method thereof
A technology of tenofovir fumarate and disoproxil, which can be applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pill delivery, etc., can solve the problem of reduced bioavailability, swallowing problems, Dissolution reduction and other problems, to achieve the effect of reducing tablet volume, reducing product cost, and reducing the amount of excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
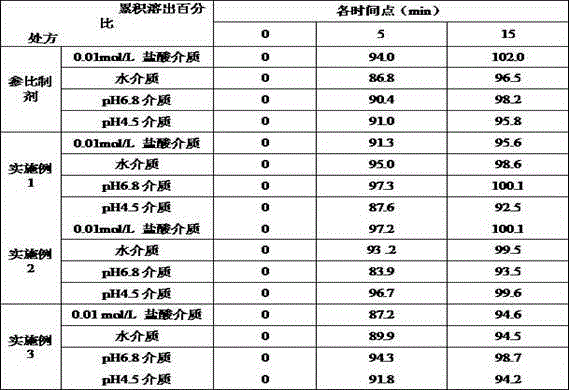
Examples
Embodiment 1
[0031] prescription:
[0032] Material composition Prescription ratio (g) Tenofovir disoproxil fumarate300.0 Microcrystalline cellulose136.4 Mannitol50.5 Sodium starch glycolate27.3 Crospovidone5.5 Micronized silica gel5.6 Pregelatinized starch20.2 Production volume 1000 pieces
[0033] Preparation method: 1) Weigh each material separately; 2) Take 400ml of purified water (10℃), add pregelatinized starch under stirring, stir well for 10 minutes, and prepare about 5% (w / v) pregel Starch suspension; 3) Mix tenofovir disoproxil fumarate, microcrystalline cellulose, mannitol, and sodium starch glycolate fully, mix and add pregelatinized starch suspension to make soft material , Extrusion granulation; 4) Dry the granules in the drying box until the moisture of the granules is not more than 1.5%, and the drying temperature is 55℃; 5) The dried granules are passed through a 20-mesh sieve to be granulated; 6) After granulation, the granules are added to crospovidone Mix with ...
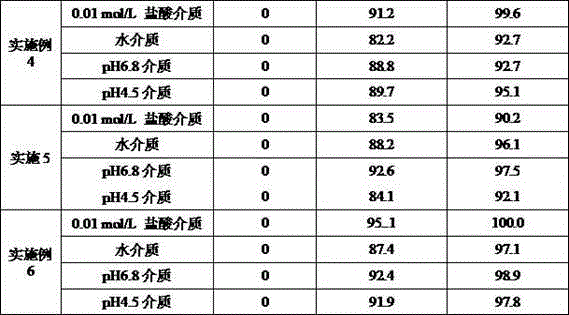
Embodiment 2
[0035] prescription:
[0036] Material composition Prescription ratio (g) Tenofovir disoproxil fumarate300.0 lactose46.0 Low-substituted hydroxypropyl cellulose12.0 Low-substituted hydroxypropyl cellulose6.0 talcum powder15.0 Pregelatinized starch21.0 Production volume 1000 pieces
[0037] Preparation method: 1) Weigh each material separately; 2) Take 100ml of purified water (20℃), add pregelatinized starch, fully stir for 45 minutes, and prepare about 20% (w / v) pregelatinized starch suspension 3) Put tenofovir disoproxil fumarate, lactose, and 12.0 g of low-substituted hydroxypropyl cellulose in a high-speed stirring granulator to mix, and then add the pregelatinized starch suspension for granulation; 4) The wet granules are dried in a fluidized bed granulator until the moisture of the granules is not more than 1.5%, and the drying temperature is 60℃; 5) The dried granules are sieved through an 18-mesh sieve; 6.0g and total talc powder; 7) Press the theoretical tablet...
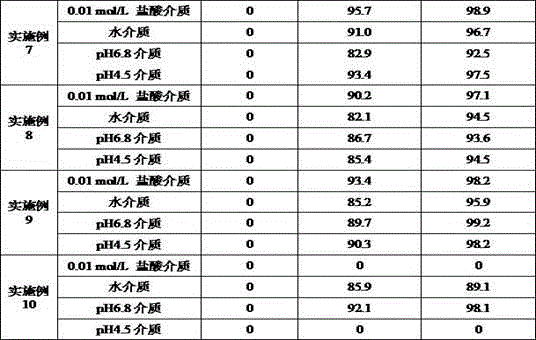
Embodiment 3
[0039] prescription:
[0040] Material composition Prescription ratio (g) Tenofovir disoproxil fumarate300.0 lactose42.8 Powdered sugar25.8 Low-substituted hydroxypropyl cellulose10.8 Sodium starch glycolate5.2 Micronized silica gel1.2 Pregelatinized starch42.8 Production volume 1000 pieces
[0041] Preparation method: 1) Weigh each material separately; 2) Take 85ml of ethanol aqueous solution [Ethanol 30% (v / v), 25℃], add pregelatinized starch under stirring, fully stir for 20 minutes, and configure it to about 25 %(W / v) pregelatinized starch suspension; 3) Pass tenofovir disoproxil fumarate, lactose, powdered sugar, low-substituted hydroxypropyl cellulose, pregelatinized starch suspension through Fluidized bed granulation machine is granulated; and dried until the moisture of the granules is not more than 1.5%, and the drying temperature is 50℃; 4) The dried granules are passed through an 18-mesh sieve to be granulated; 5) After granulation, the granules are mixed wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com