N-pheny-lamide compounds and applications thereof
A compound, phenyl technology, applied in the field of chemical medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
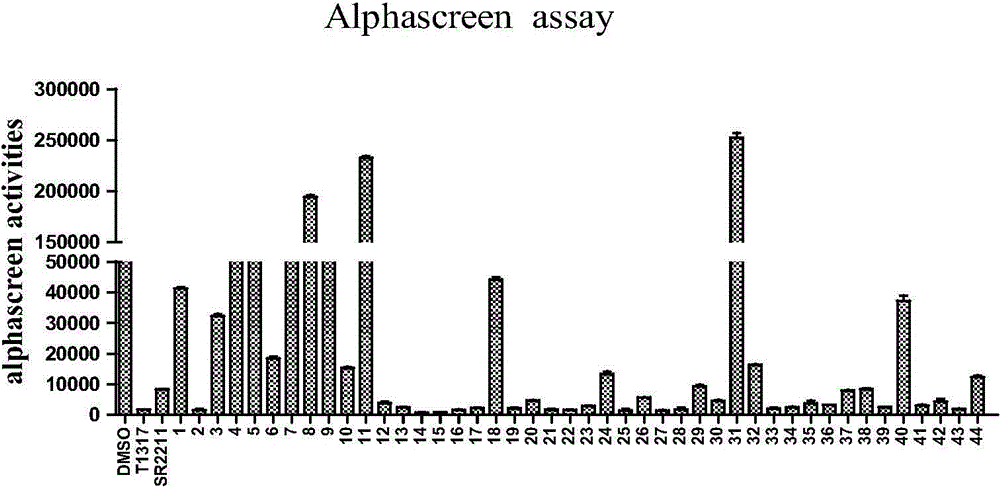
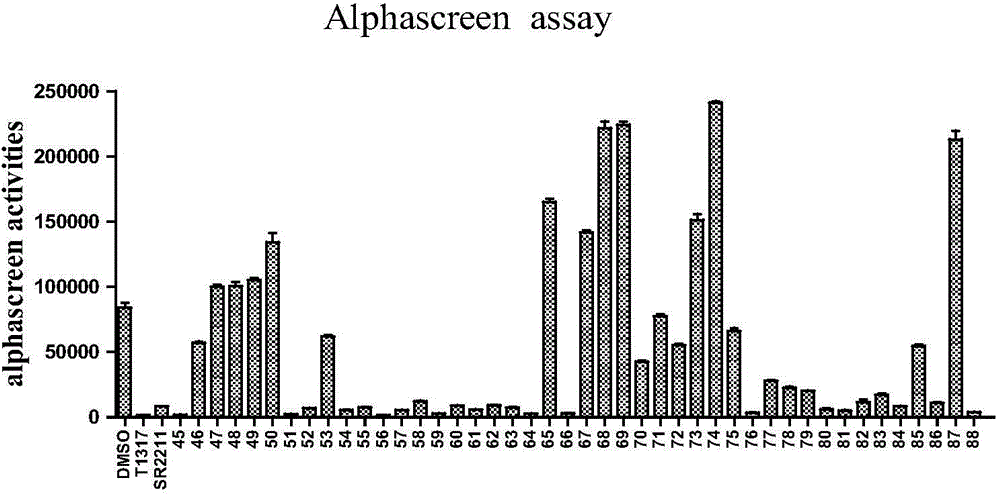
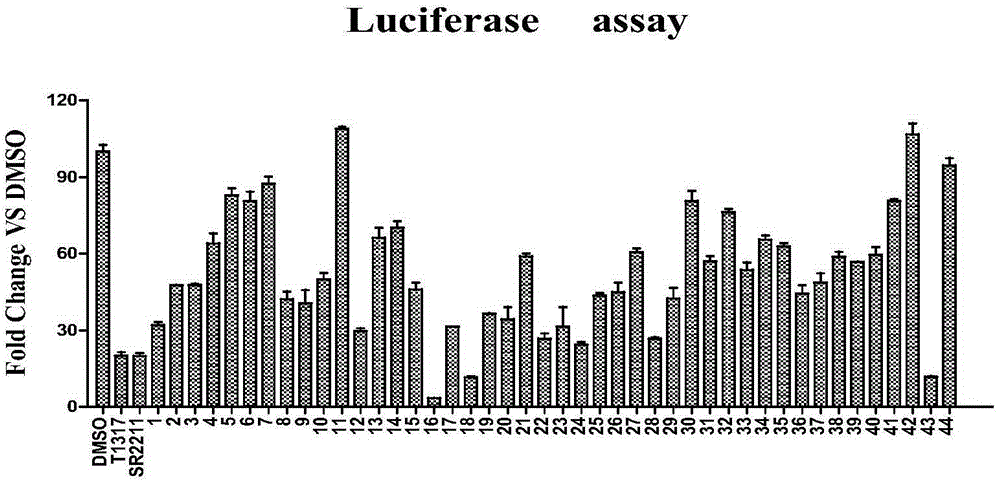
Method used
Image
Examples
Embodiment 1
[0153] 1-ethyl-N-(2-fluoro-4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)-2-oxo-1, Preparation of 2-dihydrobenzo[cd]indole-6-sulfonamide
[0154]
[0155] Step 1. Preparation of 1,3-dioxo-2,3-dihydro-1H-phenalen-2-yl 4-methylbenzenesulfonate
[0156]
[0157] Add 1,8-naphthalic anhydride (1-1) (11.9 g, 0.06 mol), hydroxylamine hydrochloride (4.18 g, 0.06 mol) and pyridine (70 mL) into a round bottom flask, and heat to reflux for 1 hour. After cooling to 80°C, 4-toluenesulfonyl chloride (22.88 g, 0.12 mol) was quickly added, and the temperature was raised to reflux for 1.5 hours. After cooling to room temperature, the mixture was poured into 200 g of ice water, and a yellow precipitate precipitated out after stirring. Stand still, filter with suction, wash with water three times, stir and wash with saturated sodium bicarbonate solution, filter with suction, wash with water three times to obtain 17g of intermediate compound 1-1 (78%).
[0158] Step 2. Preparatio...
Embodiment 2
[0174] N-(2-fluoro-4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)-4,4-dimethyl-2-oxo Preparation of -1,2,3,4-tetrahydroquinoline-6-sulfonamide
[0175]
[0176] Step 1. Preparation of 3-methyl-N-phenyl-2-propenylamine
[0177]
[0178] Take aniline (1 g, 0.01 mol), add 6 mL of pyridine, stir at 0°C, and slowly drop into 3-methylcrotonyl chloride (1.44 mL). After evacuating, use argon to keep room temperature and react for 1h. Dilute hydrochloric acid was added until there was no white precipitate, filtered with suction, the filter cake was washed with water and petroleum ether, and recrystallized from isopropanol to obtain 1.65 g of a solid (compound 2-1), with a yield of 88%.
[0179] Step 2. Preparation of 4,4-dimethyl-2-oxo-1,2,3,4-tetrahydroquinoline
[0180]
[0181] Take compound 2-1 (1.65 g, 0.009 g), add dichloromethane (20 mL), and place at 0°C. Aluminum trichloride (5 g, 0.037 mol) was added in portions. Reaction at room temperature for 2h. Was...
Embodiment 3
[0189] N-(2-fluoro-4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)-2-oxo-1-propyl-1, Preparation of 2,3,4-tetrahydroquinoline-6-sulfonamide
[0190]
[0191] Step 1. Preparation of 1-propyl-3,4-dihydroquinolin-2(1H)-one
[0192]
[0193] Take 3,4-dihydroquinolin-2(1H)-one (1g, 6.8mmol), add anhydrous DMF, stir at -5°C for 5min, add sodium hydride (0.5g, 0.02 mol), stirred for 5 min after the addition, added iodopropane (1.75 g, 0.01 mol) dropwise, and reacted overnight at room temperature. It was quenched with water and extracted three times with ethyl acetate. The organic layers were combined, washed with saturated sodium chloride, and dried over anhydrous sodium sulfate. Column chromatography: PE; EA=10:1 to obtain 1.2 g of product (compound 3-1), yield 95%.
[0194] Step 2. Preparation of 2-oxo-1-propyl-1,2,3,4-tetrahydroquinoline-6-sulfonyl chloride
[0195]
[0196] Take compound 3-1 (0.5g, 2.64mmol), add 15mL of chloroform to dissolve it, slowly add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com