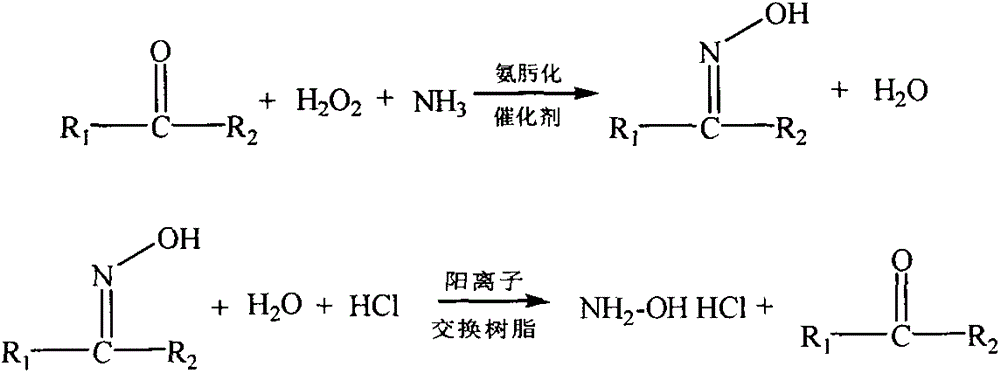
Environment-friendly synthetic method for hydroxylamine salt
A technology of green synthesis and hydroxylamine salts, applied in the fields of hydroxylamine, chemical instruments and methods, nitrogen compounds, etc., can solve the problems of complex catalyst process conditions, low conversion rate, complex process operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 42 g of ethyl orthosilicate dropwise into tetrapropylammonium hydroxide with a concentration of 20%, and stir for 2 hours to obtain solution A. And 10 grams of 20% tetrapropylammonium hydroxide was added dropwise to a mixed solution of 2 grams of butyl titanate, 0.04 grams of ammonium metavanadate and 3 grams of isopropanol, and stirred for 1 hour to obtain solution B. Solution A was added dropwise to solution B at 80°C and stirred for 5 hours. Crystallize in a PTFE hydrothermal kettle at 170°C for 60 hours, filter, and roast the solid at 2°C / min to 550°C for 5 hours to prepare the titanium-vanadium-doped ZSM-5 catalyst Ti-V-ZSM-5.
Embodiment 2
[0031] 10 g of P123 was dissolved in 250 g of 1% hydrochloric acid aqueous solution, stirred at room temperature until clear, and solution A was obtained. Mix 45 grams of ethyl silicate, 5 grams of zirconium oxychloride, 1.3 grams of ammonium metavanadate, and 2 grams of acetylacetone, and stir evenly to obtain solution B. Add solution B to solution A under stirring, and continue to stir for 15 hours, then crystallize in a 100°C PTFE hydrothermal kettle for 30 hours, filter, and heat the solid at 2°C / min to 550°C for 5 hours to prepare zirconium Vanadium doped SBA-15 catalyst Zr-V-SBA-15.
Embodiment 3
[0033] The preparation method is the same as that in Example 2, and the Ti-Mo-SBA-15 catalyst doped with titanium and molybdenum is prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com