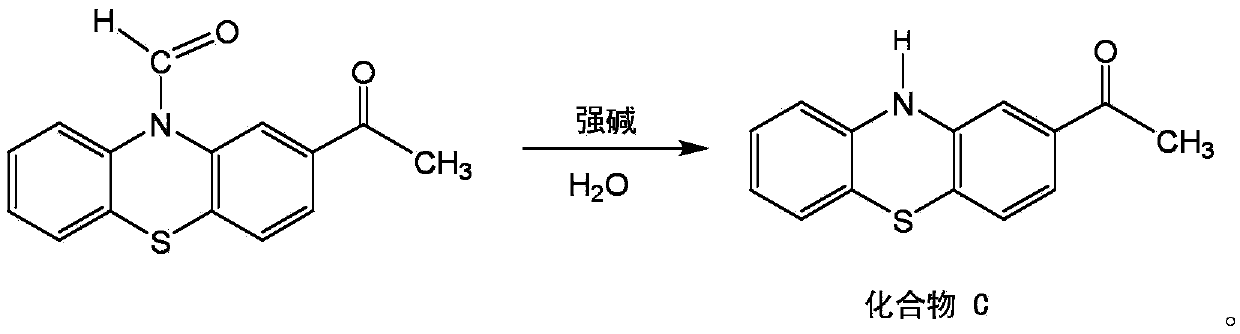
Method for synthesizing 2-acetyl phenothiazine using phenothiazine as raw material
A technique for the synthesis of acetylphenothiazine, which is applied in organic chemistry and other fields, and can solve problems such as low purity, multiple by-products, and low yield of 2-acetylphenothiazine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
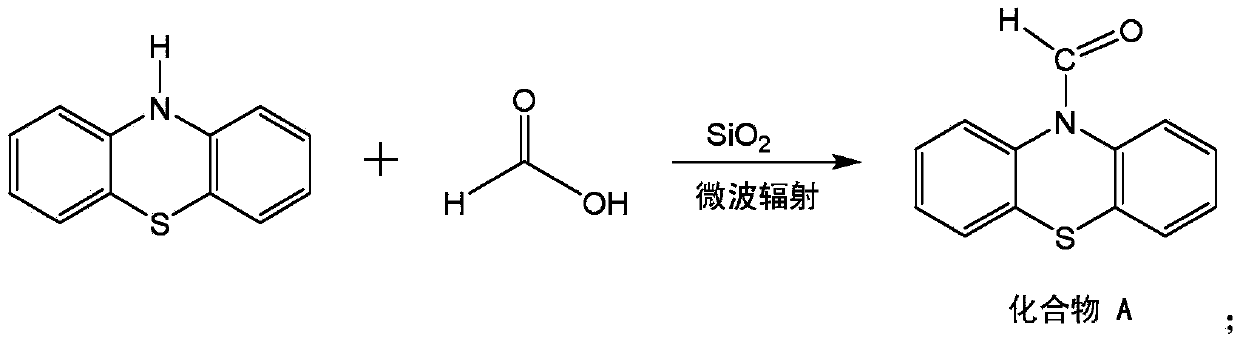
[0023] In the reaction vessel, put phenothiazine, 1.05 mass of formic acid (based on the mass of phenothiazine as 1), silicon dioxide with a quality of 0.01 (total mass of phenothiazine and diethyl malonate 1), after stirring the three evenly, the mixture was placed in a microwave oven and the power was adjusted so that the temperature of the reaction mixed raw materials was 70°C. After being heated by microwave radiation to make it react for 8 minutes, the reaction was terminated. Quenched with ice water, filtered with suction, washed the filter cake layer with water until the washing liquid was neutral, moistened with petroleum ether, washed the filter cake layer, and dried in vacuum to obtain N-formylphenothiazine, which was named compound A.
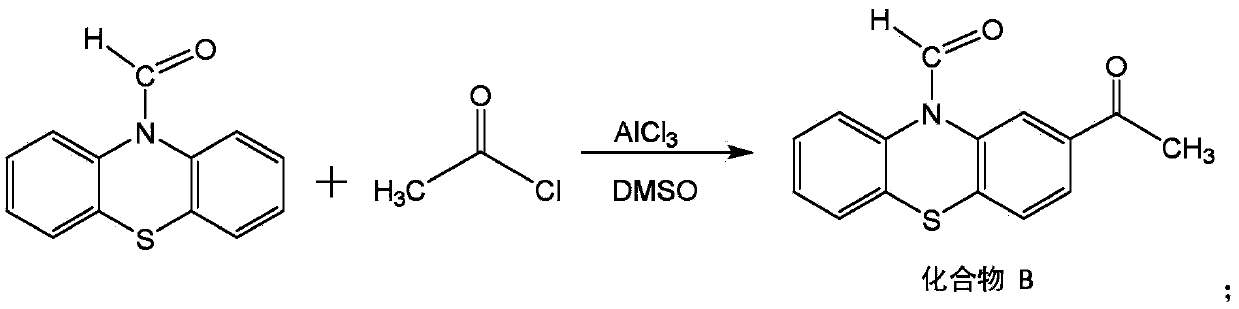
[0024] Put the above compound A in the reaction vessel, the amount of substance is 1.02 acetyl chloride (the amount of substance of compound A is 1), the amount of substance is 3 aluminum trichloride (the amount of substance of compo...
Embodiment 2
[0027] In the reaction vessel, put phenothiazine, 1.14 mass of formic acid (based on the mass of phenothiazine as 1), and a quality of 0.06 silicon dioxide (total mass of phenothiazine and diethyl malonate 1 meter), after the three are stirred evenly, the mixture is placed in a microwave oven and the power is adjusted so that the temperature of the reaction mixed raw materials is 80°C. After being heated by microwave radiation to make it react for 2 minutes, the reaction was terminated. Quenched with ice water, filtered with suction, washed the filter cake layer with water until the washing liquid was neutral, moistened with petroleum ether, washed the filter cake layer, and dried in vacuum to obtain N-formylphenothiazine, which was named compound A.
[0028] Put the above compound A in the reaction vessel, the amount of substance is 1.08 acetyl chloride (the amount of substance of compound A is 1), the amount of substance is 5 aluminum trichloride (the amount of substance of ...
Embodiment 3
[0031] In the reaction vessel, put phenothiazine, 1.10 mass of formic acid (based on the mass of phenothiazine as 1), a quality of 0.035 silicon dioxide (total mass of phenothiazine and diethyl malonate 1 meter), after the three are stirred evenly, the mixture is placed in a microwave oven and the power is adjusted so that the temperature of the reaction mixed raw materials is 75°C. After being heated by microwave radiation to make it react for 5 minutes, the reaction was terminated. Quenched with ice water, filtered with suction, washed the filter cake layer with water until the washing liquid was neutral, moistened with petroleum ether, washed the filter cake layer, and dried in vacuum to obtain N-formylphenothiazine, which was named compound A.
[0032] Put the above compound A in the reaction vessel, the amount of substance is 1.06 acetyl chloride (the amount of substance of compound A is 1), the amount of substance is 4 aluminum trichloride (the amount of substance of com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com