Formaldehyde fluorescent probe, and preparation method and application thereof
A fluorescent probe, formaldehyde technology, applied in the field of analytical chemistry, to achieve the effect of high selective detection, high sensitivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
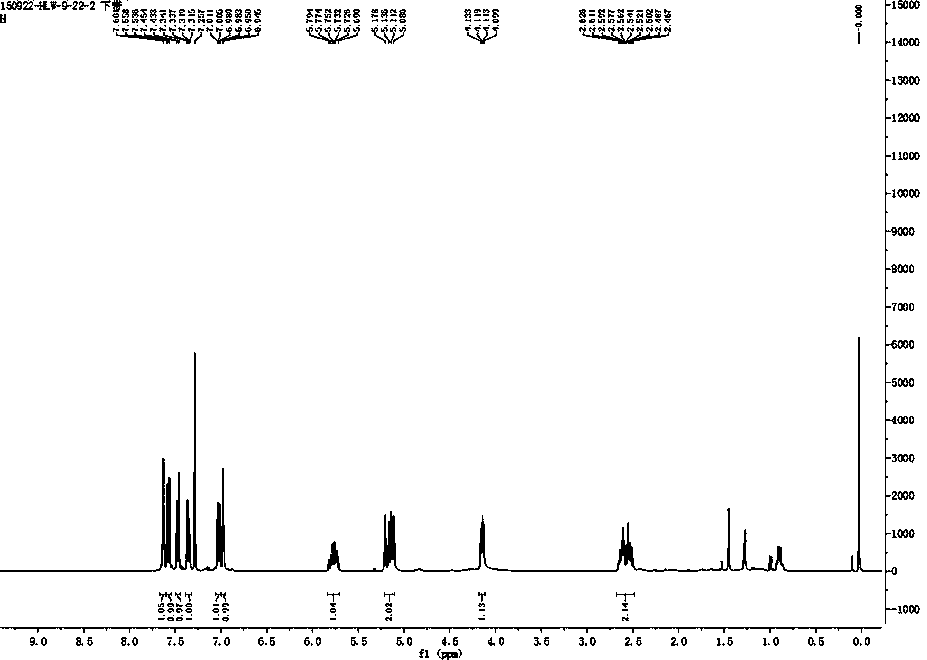
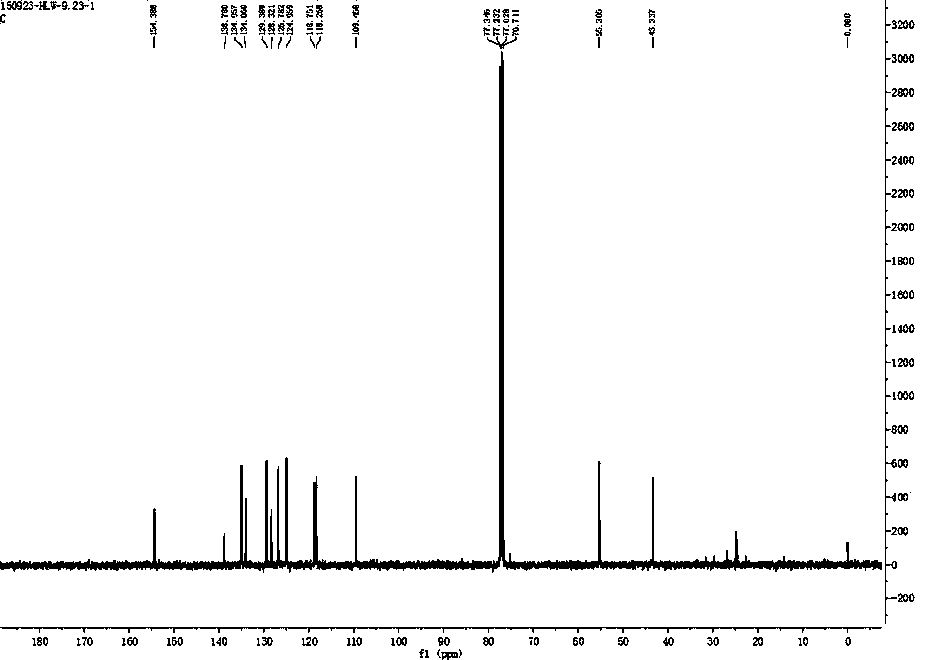
[0029] Example 1 : compound RFFP Synthesis:
[0030]
[0031] Dissolve 86mg of 6-hydroxy-2-naphthaldehyde (0.5mmol) in 5mL of methanol, add 378μL of 25% ammonia water (5mmol) in an ice bath and keep stirring for 30 minutes. Subsequently, 101 mg of allylboronic acid pinacol ester (0.6 mmol) was added, the ice bath was removed and the reaction was stirred overnight (over 10 h). After the reaction, remove the solvent from the reaction liquid by a vacuum rotary evaporator to obtain a crude product, and use a silica gel (200-300 mesh) chromatography column to purify with dichloromethane and ethanol at a volume ratio of 10:1 as the eluent , to obtain 70 mg of white solid (66% yield). 1 HNMR (400MHz, CDCl 3 ),(ppm):2.47-2.63(m,2H),4.10-4.13(t, J =6.8Hz,1H),5.09-5.18(m,2H),5.69-5.79(m,1H),6.94-6.95(1H),6.98-7.01(dd, J =8.8,2.4Hz,1H),7.31-7.34(dd, J =8.8,1.6Hz,1H),7.43-7.45(d, J =6.8Hz,1H),7.54-7.56(d, J =8.8Hz,1H),7.60(s,1H); 13 CNMR (100MHz, CDCl 3): δ (ppm): 43.34, 55...
Embodiment 2
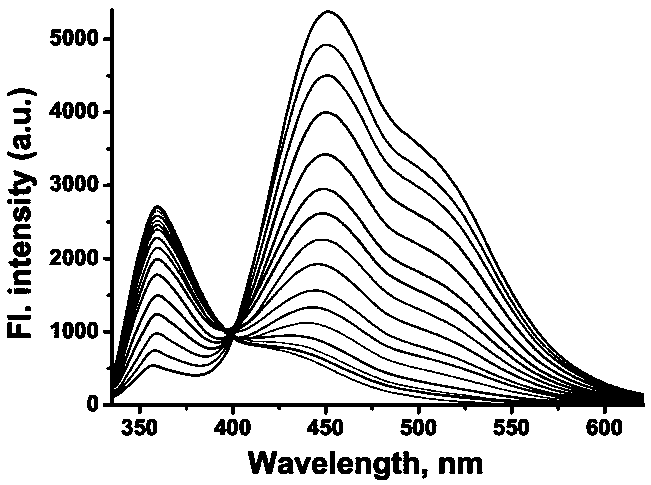
[0032] Example 2 :probe RFFP The change of fluorescence spectrum with the addition of different amounts of formaldehyde
[0033] Get the probe prepared in Example 1 RFFP Dissolve in acetone to make a concentration of 1mol / L probe mother solution (probe RFFP The concentration of formaldehyde is 1mol / L); the formaldehyde solution with a mass fraction of 37% is added to distilled water to prepare formaldehyde mother liquors with formaldehyde concentrations of 1mmol / L, 50mmol / L and 500mmol / L respectively. Take 30μL from the probe mother solution and add it to a 5mL centrifuge tube, add different equivalents (1-300eq) of formaldehyde mother solution (referring to the formaldehyde content in the added formaldehyde mother solution is the probe mother solution RFFP content of 1-300 times), diluted to 3mL with potassium dihydrogen phosphate aqueous solution (concentration 25mmol / L, pH7.4), and configured as a test solution with a probe concentration of 10μmol / L and 1% acetone. Use...
Embodiment 3
[0034] Example 3 :probe RFFP Calculation of lower limit of detection of formaldehyde
[0035] According to the fluorescence spectrogram of example 2, the fluorescence intensity at 451nm place and the fluorescence intensity ratio at 359nm place (I 451 / I 359 ) and the corresponding formaldehyde concentration. Depend on Figure 4 Visible, when formaldehyde concentration is 20-300 equivalent, I 451 / I 359 Good linearity with formaldehyde concentration. According to the formula Dl=3σ / k (Dl is the lower limit of detection, σ is the standard deviation of the intercept, and k is the slope), the lower limit of detection can be calculated as 5.53×10 -5 mmol / L.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com