Method of preparing lithium iron phosphate cathode material through hydrothermal method, and the cathode material
A technology for lithium iron phosphate and positive electrode materials, which is applied in the direction of chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve the problem of not being able to significantly improve the electronic conductivity of lithium iron phosphate positive electrode materials, shorten the distance of lithium ion transmission paths, and improve The effect cannot be continued, etc., to achieve the effect of small particle size, low cost, and anti-dropping
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In this example, LiOH·H 2 O, H 3 PO 4 and FeSO 4 ·7H 2 O is used as a raw material, and the molar ratio Li:Fe:P is 3:1:1 for batching to prepare 0.3mol of LiFePO 4 . details as follows:
[0043] LiOH·H 2 O is prepared into a 3mol / L lithium source solution, and the H 3 PO 4 Prepare a 1.5mol / L phosphorus source solution, then slowly add the phosphorus source solution into the lithium source solution to make a mixed solution A; 4 ·7H 2 O is prepared into a 1mol / L iron source solution, and added to the iron source solution to theoretically generate LiFePO 4 20wt.% of the total weight of starch was mixed to make a mixed solution B; under the action of mechanical stirring, the mixed solution B was slowly added to the mixed solution A to form a reaction system solution, and the reaction system solution was transferred to 1L volume of reaction The reaction is carried out in the kettle; the filling volume of the reaction kettle is 80%, the pH value of the reaction sys...
Embodiment 2
[0047] This example will press LiFePO 4 The 4wt.% phenolic resin of product and the carbon-coated LiFePO that embodiment one prepares 4 The product was mixed evenly by ball milling to obtain the post-treatment precursor; the post-treatment precursor was calcined at 750°C for 12 hours in a flowing nitrogen atmosphere, and cooled after calcining to obtain a spherical LiFePO with double-layer coating morphology. 4 / C / PAS.
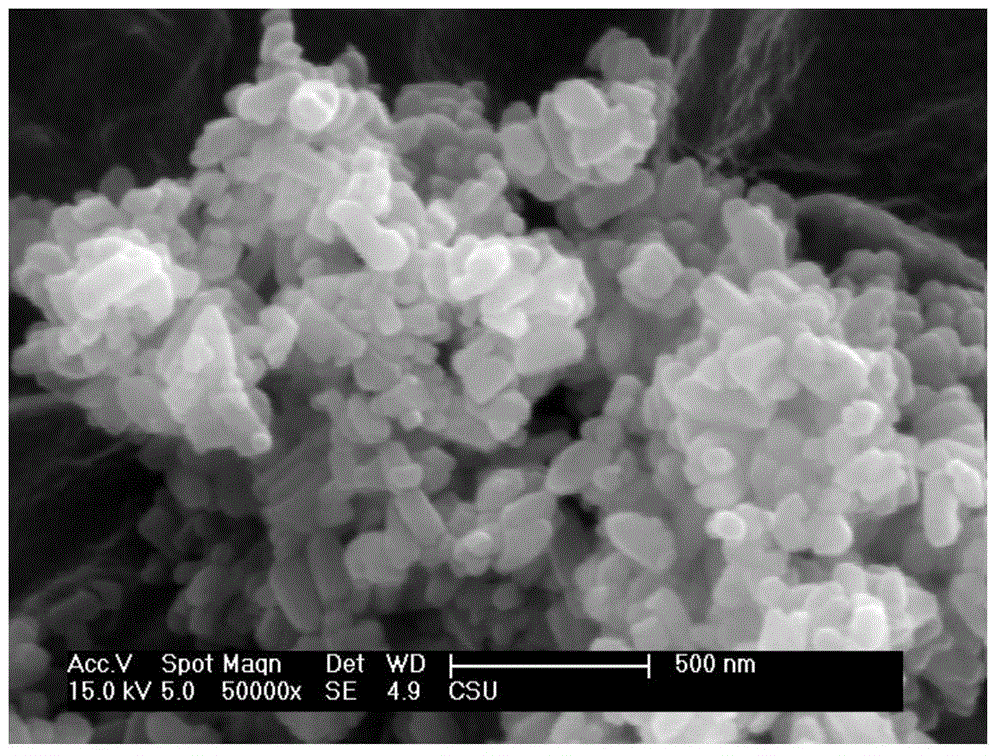
[0048] The high-resolution transmission electron microscope image of the double-coated lithium iron phosphate cathode material prepared in this example is as follows Figure 5 As shown in the figure, PASlayer is the conductive polymer coating layer, and carbonlayer is the carbon coating layer, which proves that the product has a double-layer coating effect.
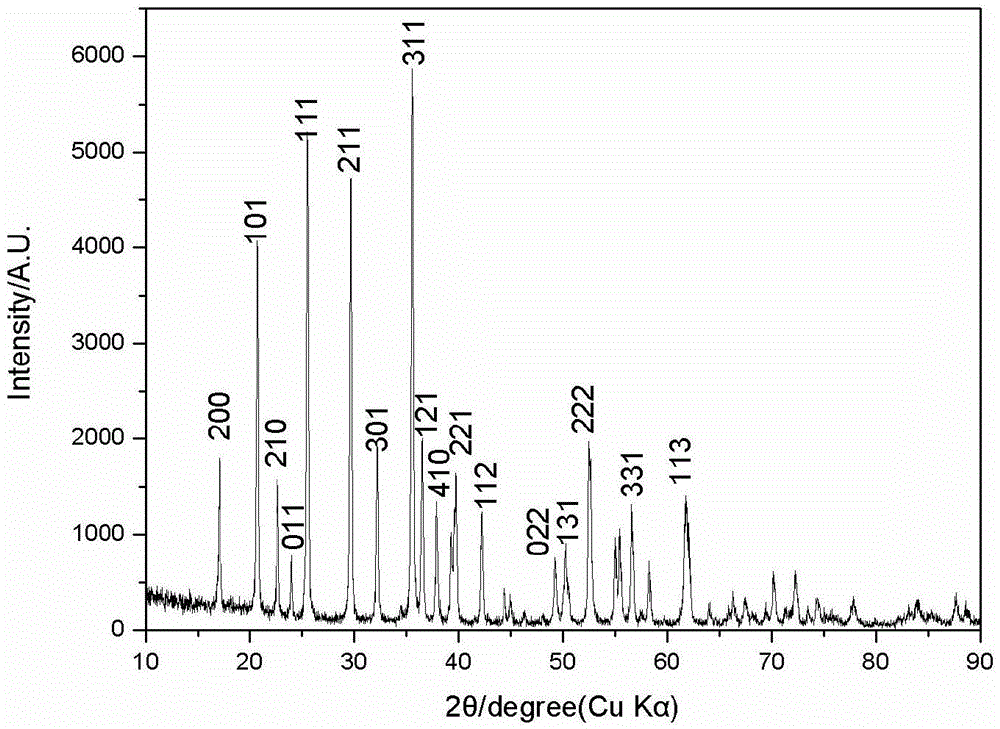
[0049] X-ray diffraction analysis and detection shows that the diffraction pattern of the double-coated lithium iron phosphate cathode material prepared in this example is consistent with the standard ...
Embodiment 3
[0054] According to the hydrothermal method of Example 1, lithium iron phosphate was mixed with 4wt.% glucose of lithium iron phosphate weight, roasted at 750 ° C for 12 hours, and ball milled after cooling to obtain a spherical carbon-coated lithium iron phosphate material. .
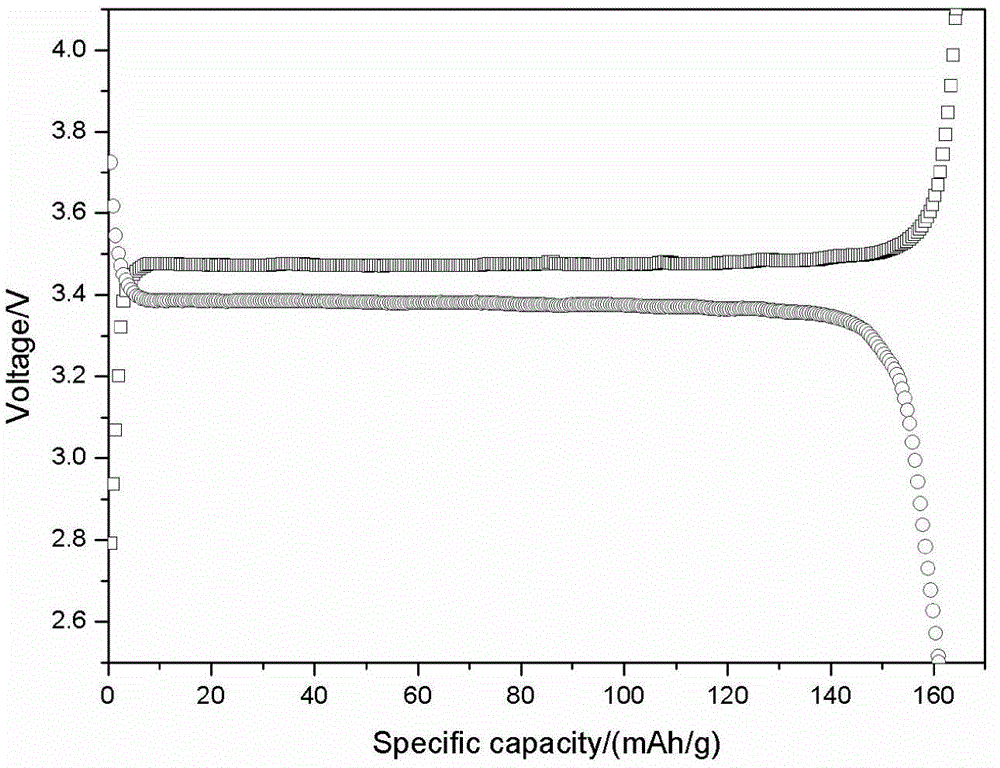
[0055] After inspection, the initial discharge capacity at 0.2C rate current is 155.8mAh / g, and it maintains 133.2mAh / g after 50 cycles at 1C, which is as high as 97.9% relative to the initial capacity retention at 1C.
[0056] It can be seen that the effect of invisible carbon coating on the products prepared by the hydrothermal method is relatively poor. The main reason is that the invisible carbon coating layer is eroded by the electrolyte during the electrochemical process of the battery and is easy to fall off, resulting in poor battery performance. Therefore, comparatively speaking, the double-layer coating effect of Example 2 is better, and both the initial charge and discharge capacity and cycl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com