A kind of method for preparing obeticholic acid type 1
A technology for obeticholic acid and preparation process, which is applied in the field of preparing obeticholic acid type 1, can solve problems such as unfavorable commercial production, increased operation steps, prolonged production cycle, etc., and achieves shortened synthesis steps, short production cycle, and low cost. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of (E)-3α,7α-dihydroxy-6-ethylene-5β-cholan-24-oic acid
[0047] Add (E)-3α-hydroxyl-6-ethylene-7-ketone-5β-cholan-2 4-acid (400.0g, 0.96mol), sodium hydroxide (192.0g, 4.8mol) in the reaction flask and water (4.0 L), heated to 95° C. under stirring, added sodium borohydride (36.3 g, 0.96 mol), and reacted for 3 hours, and TLC tracked that the reaction of the raw materials was complete. After the reaction, cool to 40°C, add ethyl acetate (6.0L) and water (4.0L), adjust the pH to 3 with 37% hydrochloric acid, separate the layers, dry the organic phase with anhydrous sodium sulfate for 2 hours, filter, and filter the filtrate at 40 °C and evaporated to dryness under reduced pressure to obtain a white solid, namely (E)-3α,7α-dihydroxy-6-ethylene-5β-cholan-24-acid (394.6g, purity: 99.6%, yield: 98.2%) .
Embodiment 2
[0049] Preparation of (E)-3α,7α-dihydroxy-6-ethylidene-5β-cholane-24-oic acid methyl ester
[0050] Add (E)-3α-hydroxy-6-ethylidene-7-keto-5β-cholan-24-oic acid (50.0 g, 0.12 mol), anhydrous methanol (100 mL) and methanesulfonic acid ( 0.5 g), the temperature was raised to 60° C. under stirring, and the reaction was carried out for 2 hours. TLC traced that the reaction of the raw materials was complete. After the reaction, water (75mL) was added to the reaction mixture, cooled to 5-10°C and stirred for 3 hours, filtered, the filter cake was washed with a small amount of water and methanol, and the filter cake was vacuum-dried at 50°C to obtain a white solid, namely (E)- 3α,7α-Dihydroxy-6-ethylene-5β-cholane-24-oic acid methyl ester (45.8 g, purity: 99.5%, yield: 88.2%).
Embodiment 3
[0052] Preparation of obeticholic acid type 1
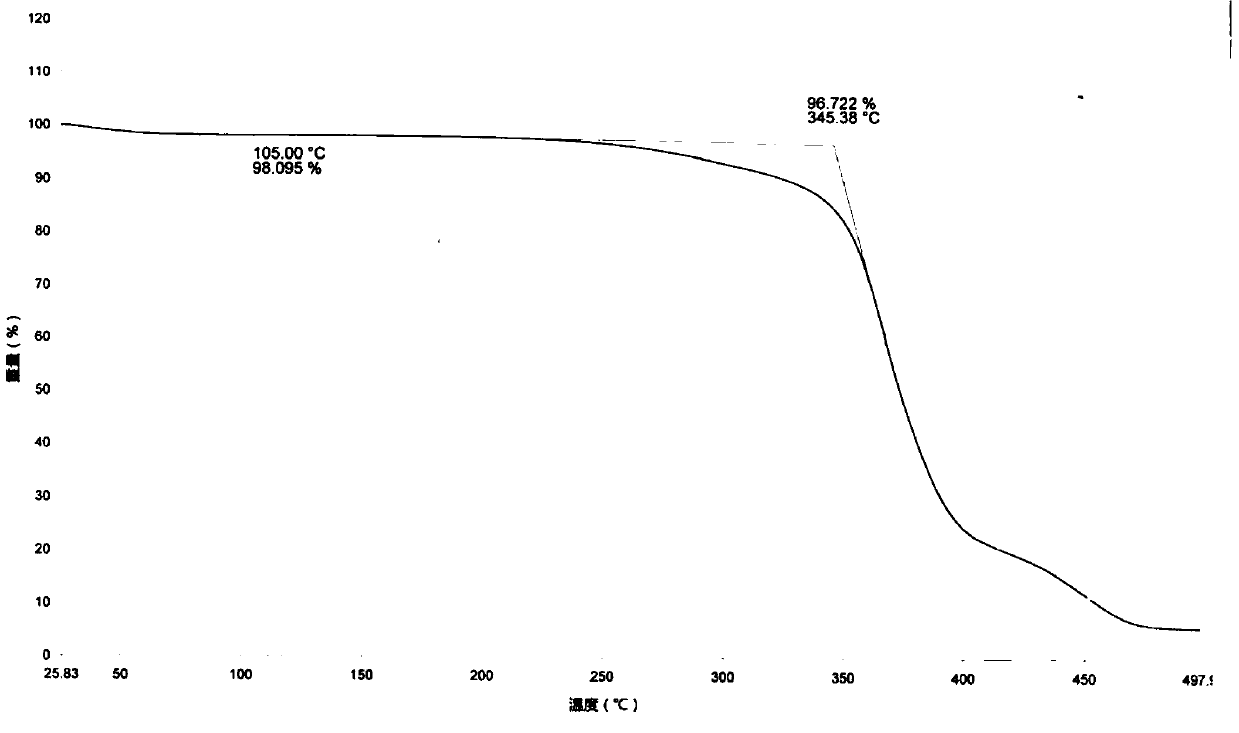
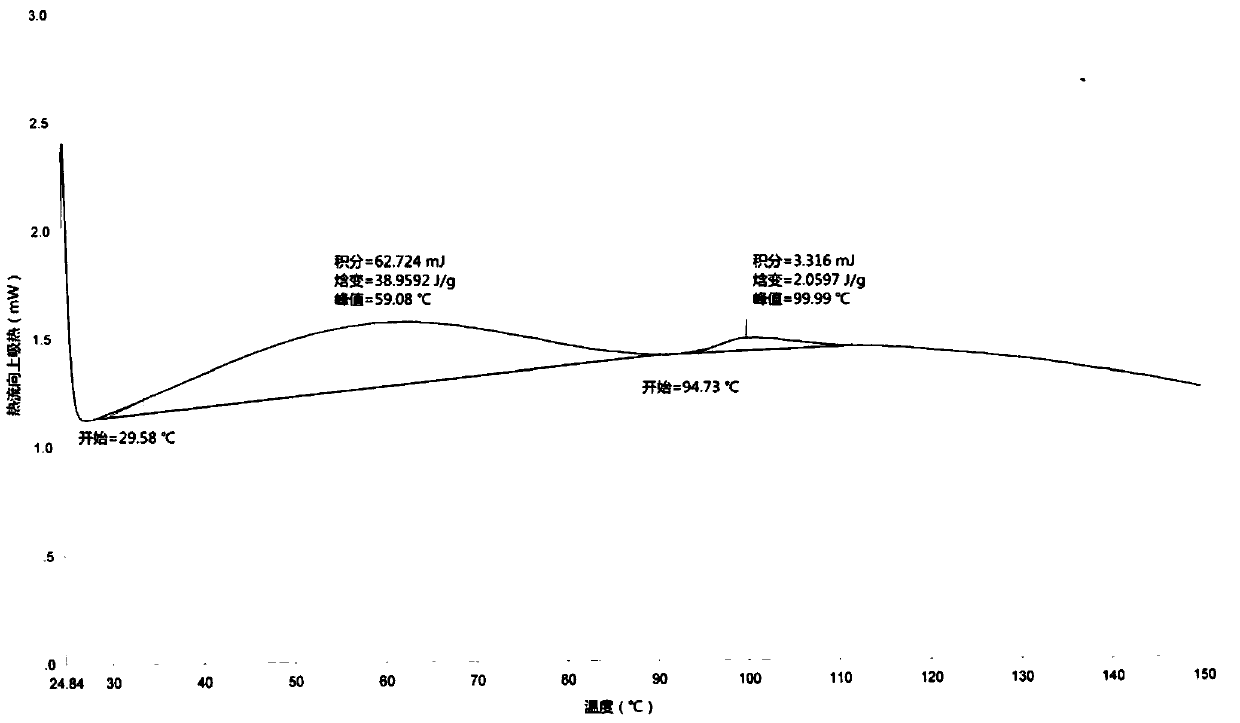
[0053] Add (E)-3α, 7α-dihydroxy-6-ethylene-5β-cholan-24-acid (50.0g, 0.12mol), sodium hydroxide (9.6g, 0.24mol) and Water (400mL) and 5% palladium on carbon (5.0g), the reaction mixture was heated to 80°C under a pressure of 1-3 atmospheres, and after 3 hours of hydrogenation reaction, it indicated that hydrogen gas was no longer absorbed. The reaction solution was cooled to 50°C, filtered, and water (750 mL) was added to the filtrate, and the temperature was raised to 50°C. Add 1.0 mol / L hydrochloric acid solution dropwise to adjust the pH to 3.0, continue to heat and stir for 30 minutes, cool to 10°C and stir for crystallization for 2 hours, filter, and vacuum-dry the filter cake at 50°C for 3 hours to obtain a white solid, namely obeticholic acid 1 Form (42.5g, purity: 99.3%, yield: 84.2%).
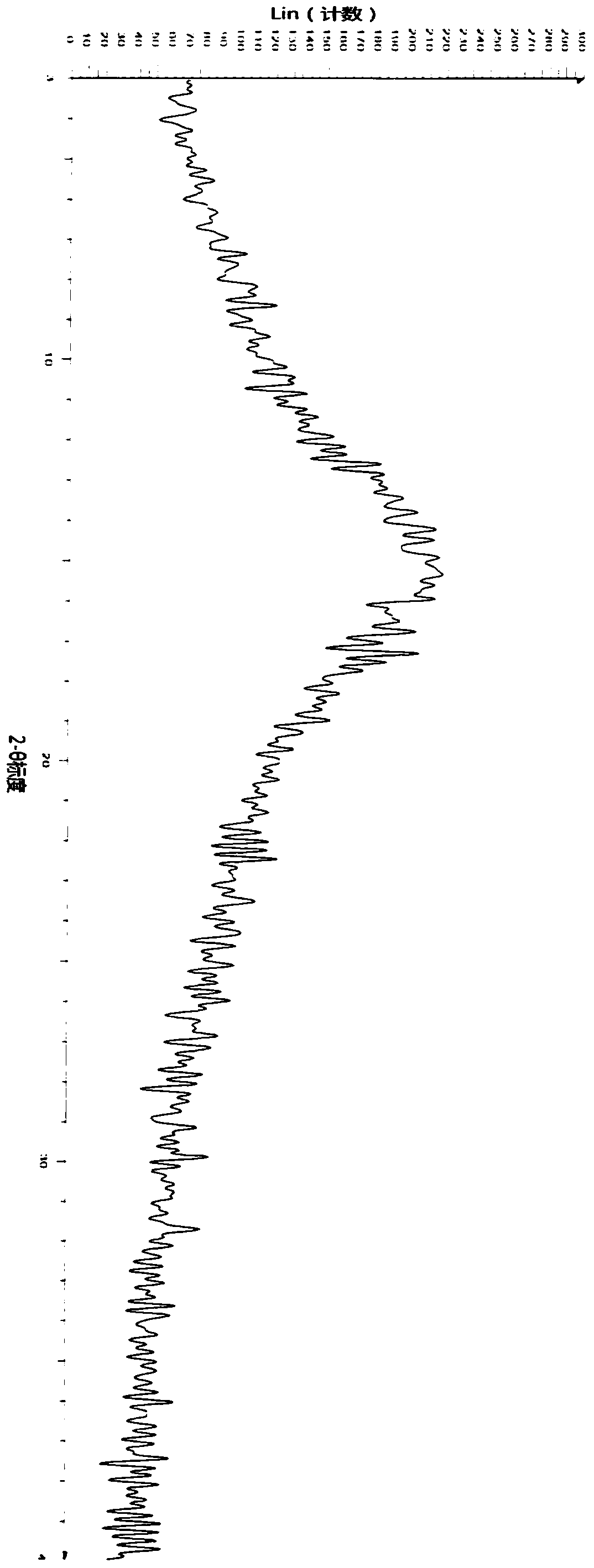
[0054] Present embodiment sample X-ray powder diffraction pattern (XRPD) sees attached figure 1 , the X-ray powder diffraction patter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com