A kind of preparation method and composition of original developed quality cephradine
A technology of cephradine and composition, which is applied in the field of preparation of the original developed quality cephradine, can solve problems such as potential safety hazards, harsh reaction conditions, combustion and explosion, etc., and achieves the effects of simple and easy preparation method, low raw material cost, and avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
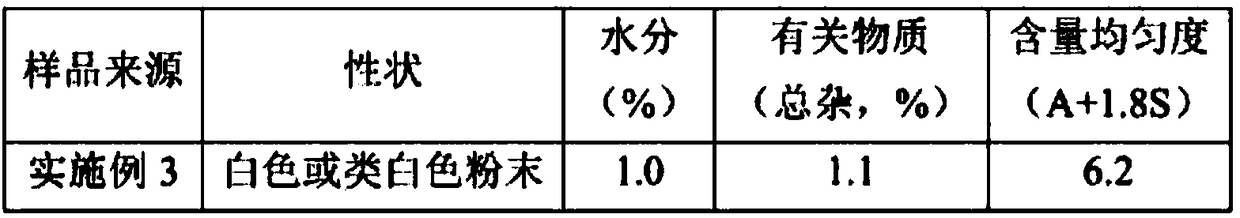
[0028] Embodiment 1 (preparation of cefradine)
[0029] (1) Add 100g of 7-ADCA, 500ml of dichloromethane, blow in nitrogen, stir, add 120ml of N-methylacetamide, heat to 45°C, add slowly and then slowly add 90g of 1,8-diazabicyclo[ 5,4,0] Undec-7-ene, constant temperature reflux reaction for 1.5 to 2 hours to obtain 7-ADCA protected by DBU;
[0030] (2) Add 120g of dihydrophenylglycine acid chloride hydrochloride to 300ml of dichloromethane, cool down to -20°C~-30°C; slowly add 7-ADCA protected by DBU at 0°C, and keep it at -5°C~ -10℃, condensation reaction for 2 hours;
[0031] (3) Add 500ml of water to the hydrolysis tank, cool to 2°C, add the reaction solution after the condensation reaction in the previous step and 140ml of concentrated hydrochloric acid into the hydrolysis tank, stir at 10°C for 15min, let stand, separate the organic phase layer and wait Use; Aqueous layer is heated to 38 ℃ rapidly, distills under vacuum condition simultaneously, to go out residual dich...
Embodiment 2
[0033] Embodiment 2 (the recovery of cephradine)
[0034] Combine the organic phase layer and the filtrate in Example 1, pass the combined solution through a 0.45 μm microporous filter membrane, then pump it into a polyamide membrane device for processing, first concentrate to 65% to 75% of the original volume of the combined solution, and then Add 1-2 times the current volume of water, and then concentrate to 15% to 25% of the original volume of the combined solution to obtain the concentrated solution 1, and the permeate is ready for use;
[0035] The permeate is pumped into a polyamide membrane device for treatment, and concentrated to 10% to 15% of the original volume of the permeate to obtain a concentrated solution 2;
[0036] Concentrate 1 and concentrate 2 are combined, and cephradine is extracted by β-naphthol complexation.
Embodiment 3
[0037] Embodiment 3 (preparation of composition)
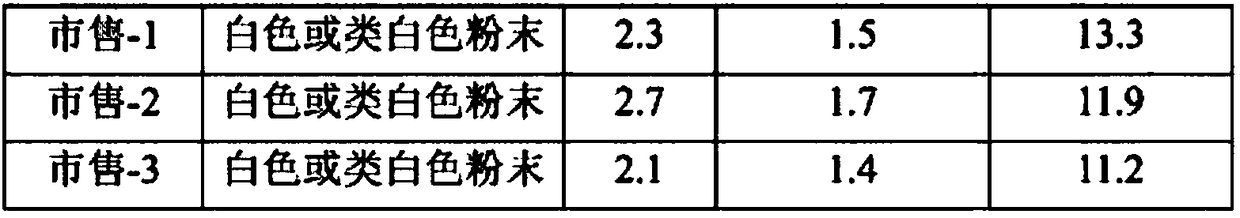
[0038] Get L-arginine, the cephradine prepared by embodiment 1, by weight, according to the proportioning of 400 parts of L-arginine, 1000 parts of cephradine, place in water for injection, stir and make it dissolve. Spray drying in QZR-5 spray dryer, inlet temperature 105℃~115℃, outlet temperature 50℃~55℃, feed rate 25~40ml / min, nozzle pressure 0.25~0.40kgf / cm 2 , collect and mix powder with the cyclone separator to get the finished product. Get the finished product, pack in subpackages of 0.765 grams (equivalent to 0.5 grams of cephradine), crimp and pack afterwards to get final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com