Application of polypeptide chelated calcium in preparing transdermic absorbent
A technology of polypeptide chelating calcium and preparations, applied in skin care preparations, tripeptide ingredients, skin diseases and other directions, can solve the problems of difficult absorption of oral calcium, difficult absorption of calcium ions, difficult percutaneous absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
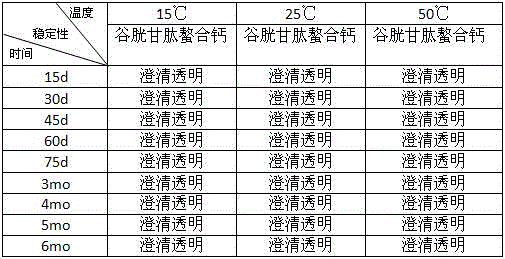
[0050] Preparation of polypeptide chelated calcium: In this experiment, glutathione was used as a ligand, and calcium chloride, calcium carbonate, calcium phosphate, calcium hydrogen phosphate, calcium silicate, calcium lactate, calcium propionate, calcium citrate, One of calcium gluconate is used as a calcium source to provide central ion calcium to prepare polypeptide chelated calcium for further experimental research, as follows:
[0051] 1. Preparation of glutathione chelated calcium
[0052] 1) Weigh 100g of glutathione, dissolve it in a three-necked flask with 350ml of deionized water, and heat it in a water bath to a constant temperature of 60°C until glutathione is completely dissolved;
[0053] 2) Weigh 100g of calcium lactate, completely dissolve in enough water, and adjust the pH to 8.0;
[0054] 3) Slowly add the calcium lactate solution prepared in 2) to the glutathione solution in 1) drop by drop, stir gently while adding the calcium lactate solution, and keep a...
Embodiment 2
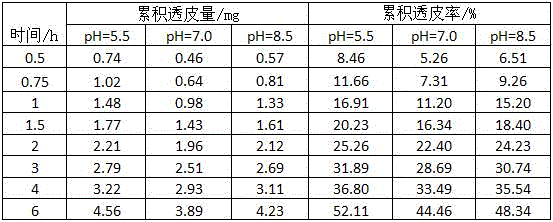
[0066] This experiment compared the transdermal absorption effect of polypeptide chelated calcium and amino acid chelated calcium, inorganic calcium and organic acid calcium. In the test, isolated mouse skin was used, and the receiving solution was 0.9% sodium chloride aqueous solution. The test time was 10h. The polypeptide chelated calcium selected in the test is the glutathione chelated calcium prepared in Example 1, the amino acid chelated calcium is glutamic acid chelated calcium, the inorganic calcium is calcium chloride, and the organic acid calcium is calcium gluconate , the specific test method is to prepare glutathione chelated calcium, glutamate chelated calcium, calcium chloride, and calcium gluconate into solutions with the same mass concentration, and take the effective test area of mouse skin as diameter d=1.5cm Take the same volume of three solutions and apply them evenly on the surface of mouse skin, test and compare glutathione chelated calcium, glutamate c...
Embodiment 3
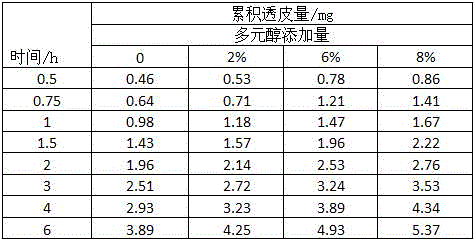
[0071] In this experiment, the glutathione chelated calcium prepared in Example 1 was used as the test sample, and the effects of different concentrations of glutathione chelated calcium solutions on the transdermal effect were compared. In the test, isolated mouse skin was used, the receiving solution used was 0.9% sodium chloride aqueous solution, and the test time was 6 hours. Glutathione chelated calcium was formulated into solutions of different mass concentrations, and the effective test area of the mouse skin was a circular size with a diameter of d=1.5cm, and glutathione chelated calcium solutions of various concentrations with the same volume were taken Apply evenly on the surface of mouse skin, test and compare with franz diffusion cell transdermal tester, the specific results are shown in Table 2 below:
[0072] Table 2. Transdermal effects of different concentrations of glutathione chelated calcium
[0073]
[0074] As can be seen from the above table, the tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com