Ph-responsive nanometer drug delivery system based on dendrimers modified by short-chain alkane and preparation method and application of drug delivery system
A nano-drug delivery system and dendrimer technology, applied in the field of nano-drug delivery system, can solve the problems of cell tissue damage, uncontrollable release process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
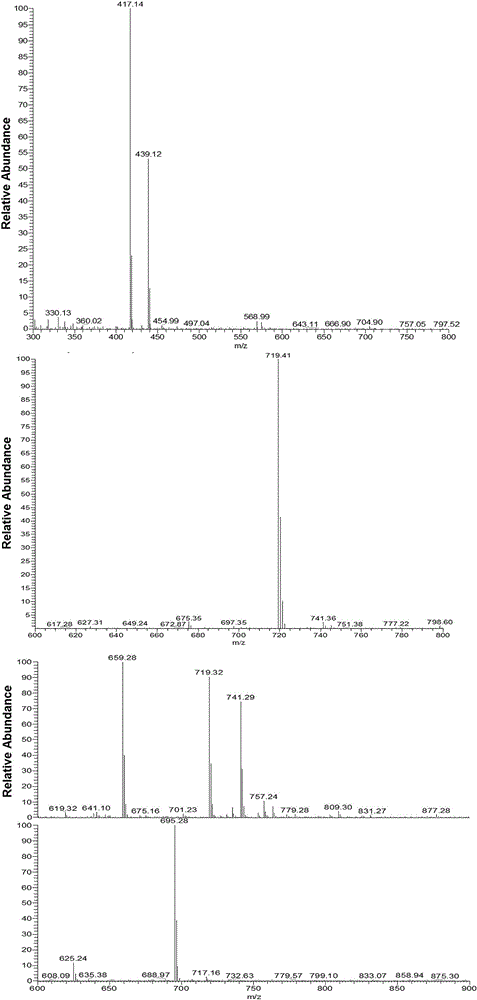
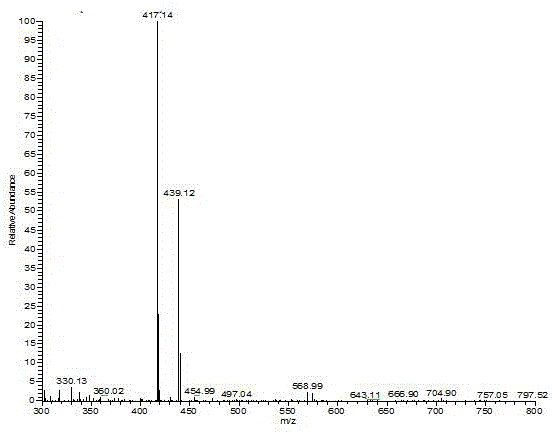
[0050] Add H-Lys-OMe·2HCl (2.5g), Boc-L-Lys(Boc)-OH (12.81g), EDC?HCl (7.0g) and HOBT (5.0g) into the reaction flask and redistill with 80ml dissolved in dichloromethane, under the protection of nitrogen, add DIPEA10.2ml, react at room temperature for 48h, rotary steam, add chloroform to dilute, and then use saturated NaHCO 3 solution, washed with HCl (1M) and saturated NaCl solution, anhydrous MgSO 4 After drying overnight, the product 2 (MeO-D-Boc) was obtained by column separation.
[0051] Second-generation lysine dendrimers (MeO-D):
[0052] Under nitrogen protection, MeO-D-Boc (1.82 g, 2 mmol) was dissolved in 6 ml CH 2 Cl 2 , then added TFA7ml, reacted at room temperature for 7h, rotary steamed, diethyl ether precipitated, removed diethyl ether to obtain a white powder (MeO-D).
[0053] Short-chain alkane-modified second-generation lysine dendrimers (MeO-DH):
[0054] Under the protection of nitrogen, dissolve MeO-D (2.4mmol) in 30ml of anhydrous methanol, add exce...
Embodiment 2
[0060] Example 3: Preparation of amphiphilic compound (HA-DH)
[0061] The hydrazide group of Hydrazine-DH reacts with the aldehyde group of the hyaluronic acid derivative. Under the protection of nitrogen, the Hydrazine-DH and HA-CHO obtained in the above reaction were dissolved in the buffer solution of pH 7.4 at the same ratio, and reacted at 40° C. for 24 hours. After the reaction was completed, it was dialyzed in water and freeze-dried to obtain the product (HA-DH).
Embodiment 3
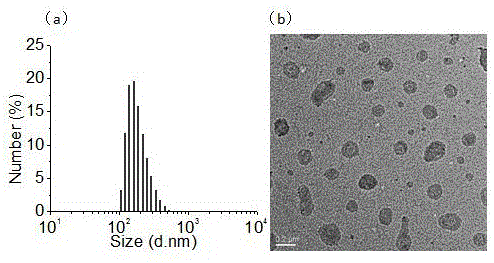
[0062] Embodiment 4: the critical self-assembly concentration of HA-DH
[0063] Using pyrene as a fluorescent probe, the critical self-assembly concentration of HA-DH was investigated by fluorescence spectroscopy. First dilute the solution of pyrene in acetone to 1.2*10 -6 M, take 1ml and add it to a 10ml glass bottle, put it in a vacuum drying oven to remove acetone, then add 2ml of materials with different concentrations, heat at 60°C for 1h, cool at room temperature and place in a dark place overnight. Taking 390nm as the emission, scanning the excitation spectrum of 300-360nm, the critical micelle concentration was obtained by the function of the ratio of I338 / I334 in the fluorescence spectrum of pyrene and logC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com