Beta-galactosidase mutant and related biological material and application thereof
A technology of galactosidase and biomaterials, applied in β-galactosidase mutants and related biomaterials and application fields, can solve problems such as narrow application range, poor heat resistance, and inability to meet the needs of the food industry, and achieve cost Inexpensive, short incubation time, good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1, the acquisition of β-galactosidase mutant and its coding gene
[0053] In order to improve the heat resistance of wild-type β-galactosidase (named β-Gal-1), the following positions of the amino acid sequence of β-Gal-1 were mutated: the amino group at position 523 was mutated from serine to Arginine (S523R); deletion of amino acid residues 512-533 (Del(512-533)); mutation of amino acid 537 from lysine to asparagine (K537N); amino acid 414 from glycine Mutation to cysteine (G414C). Only amino acid 523 (S253R) was selectively mutated. The amino acid residues at position 512-533 were selectively deleted (Del(512-533)) and the amino acid at position 537 was mutated (K537N). Amino acid residues 512-533 were selectively deleted (Del(512-533)) and amino acids 537 and 414 were mutated (K537N and G414C). The specific wild-type β-galactosidase mutants are β-Gal-2, β-Gal-3 and β-Gal-4, respectively, and the specific protein mutation sites are shown in Table 1. ...
Embodiment 2
[0061] The preparation of embodiment 2, β-galactosidase
[0062] 1. Construction of β-galactosidase recombinant expression vector
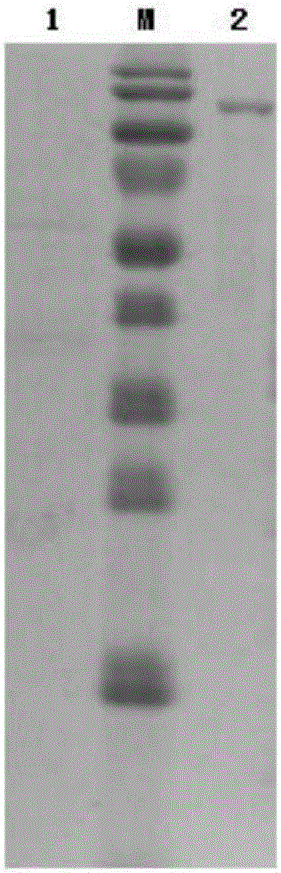
[0063] Use NcoI and KpnI to double-enzyme digest the DNA molecule shown in SEQIDNo.8, the DNA molecule shown in SEQIDNo.2, the DNA molecule shown in SEQIDNo.4 and the DNA molecule shown in SEQIDNo.6 respectively, and obtain the DNA molecules containing β-Gal respectively - DNA fragments of genes encoding 1, β-Gal-2, β-Gal-3 and β-Gal-4, will contain β-Gal-1, β-Gal-2, β-Gal-3 and β-Gal The DNA fragment of the coding gene of -4 was recovered and purified.
[0064] The purified DNA fragments containing the coding genes of β-Gal-1, β-Gal-2, β-Gal-3 and β-Gal-4 were combined with the lactic acid bacteria vector pNZ8149 obtained by NcoI and KpnI double enzyme digestion of lactic acid bacteria vector pNZ8149 The large fragments were ligated, transformed into DH5α competent cells, and positive clones were selected by restriction enzyme digestion. The r...
Embodiment 3
[0070] Embodiment 3, the biological characteristic detection of β-galactosidase
[0071] 1. Determination of enzyme activity of β-galactosidase
[0072] The enzyme activities of the four β-galactosidases in Example 2 were measured by measuring the amount of nitrophenol released by hydrolyzing the substrate ONPG. The four purified β-galactosidases obtained in Example 2 were diluted 100 times. Take 100 μL of the diluted β-galactosidase dilution and 1.8 mL of 50 mM citric acid-disodium hydrogen phosphate buffer (pH6.5), preheat at 37 ° C for 5 min, and then add 100 μL of 20 mM ONPG for reaction. After 10 min of reaction, add 1 mL of Na 2 CO 3 solution (1mol / L), the reaction terminated.
[0073] The release of nitrophenol was quantitatively detected by measuring the A420 light absorption value. The experiment was repeated 3 times. The unit of β-galactosidase activity is defined as the amount of enzyme required to decompose the substrate to generate 1 μmol of nitrophenol per ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com