Pharmaceutical composition for improving osteoarticular diseases, disintegrating tablets and preparation method of pharmaceutical composition
A technology for bone and joint diseases and compositions, which is applied in the field of pharmaceutical compositions for improving bone and joint diseases, and can solve problems such as insignificant drug effects and slow onset of bone and joint diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
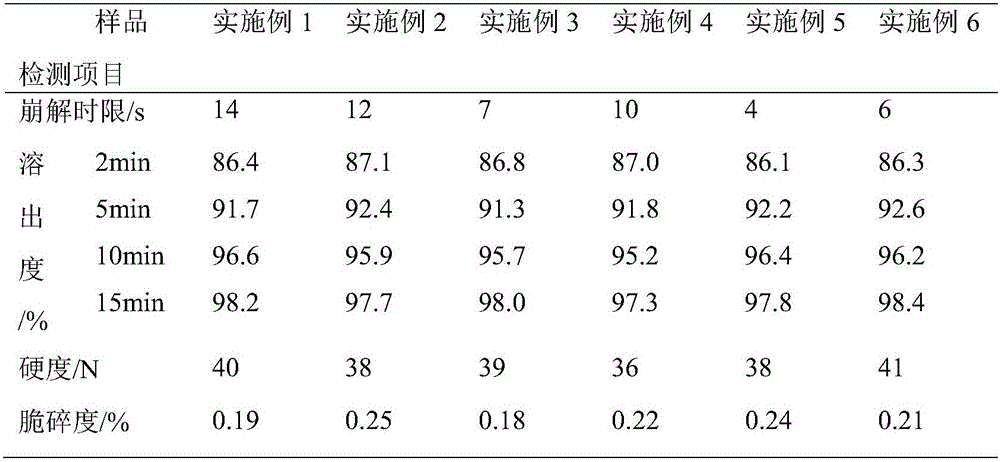
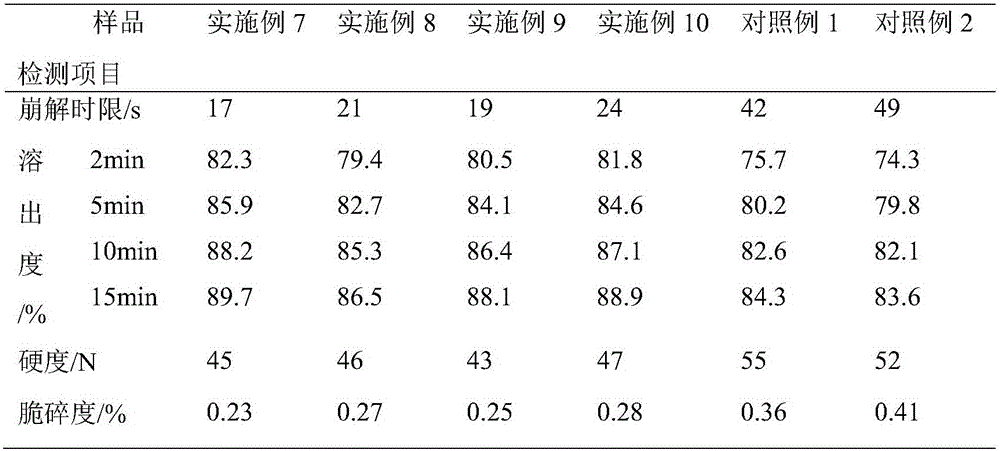
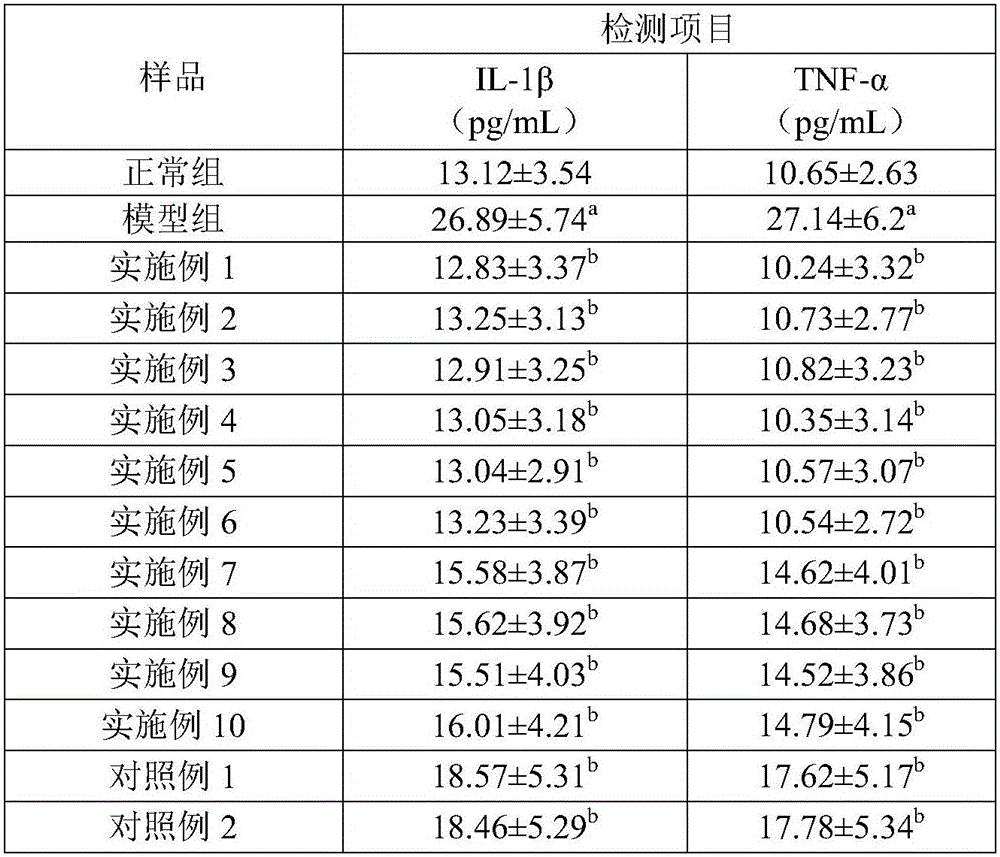
Embodiment 1
[0039] Formula: 95 parts of glucosamine, 15 parts of taurine, 20 parts of microcrystalline cellulose, 15 parts of crospovidone, 10 parts of xylitol, 5 parts of hydroxypropyl methylcellulose, 2.8 parts of talc, micronized 2.8 parts of silica gel, 1.4 parts of magnesium stearate.
[0040] Process: Weigh each raw material according to the prescription, crush glucosamine, taurine, microcrystalline cellulose, xylitol and partially cross-linked povidone, mix them evenly, put them in a mixer, and add hydroxypropyl methylcellulose , and stir evenly to make a soft material. Extrude the soft material through the sieve to make granules, or pass the soft material through the sieve twice to make the granules more uniform and less fine powder. Place the wet granules in a desiccator to remove excess moisture from the granules. Arranging the dried granules to disperse the agglomerated and cohesive granules to obtain granules of uniform size. After the granulation is completed, add talcum p...
Embodiment 2
[0042] Formula: 95 parts of glucosamine, 25 parts of taurine, 20 parts of microcrystalline cellulose, 15 parts of crospovidone, 10 parts of xylitol, 8 parts of hydroxypropyl methylcellulose, 2.8 parts of talcum powder, micronized 2.8 parts of silica gel, 1.4 parts of magnesium stearate.
[0043] Disintegrating tablets were prepared according to the same preparation process as in Example 1.
Embodiment 3
[0045] Formula: 105 parts of glucosamine, 15 parts of taurine, 25 parts of microcrystalline cellulose, 18 parts of crospovidone, 16 parts of xylitol, 7 parts of hydroxypropyl methylcellulose, 4 parts of talc, micronized 4 parts of silica gel, 2 parts of magnesium stearate.
[0046] Disintegrating tablets were prepared according to the same preparation process as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com