An N-acetylglutamate kinase mutant with improved catalytic efficiency
A glutamate kinase and catalytic efficiency technology, applied in the field of N-acetylglutamate kinase mutants, can solve the problems of weak catalytic activity, poor thermal stability, and low specific enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Construction of embodiment 1 mutant expression plasmid and acquisition of recombinant E.coli BL21 strain
[0020] According to the argB gene sequence of Corynebacterium crenatum SYPA5-5, design the two primers of the N-acetylglutamate kinase coding gene, and then design the PCR point mutation intermediate primer according to the amino acid site to be mutated:
[0021] The mutant gene was amplified in vitro by overlap extension PCR.
[0022] The primers used for site-directed mutagenesis were:
[0023] PargB F: 5'-CGCGAATTCATGAATGACTTGATCAAAG-3' (EcoRI)
[0024] PargB R: 5'-CGCGTCGACTTACAGTTCCCCATCCTTG-3'(SalI)
[0025] Parg B I74V Fm: 5'-CGGTGGTGGACCTCAGGTTTCTGAGATGC-3'
[0026] Parg B I74V Rm: 5'-GCATCTCAGAAACCTGAGGTCCACCACCG-3'
[0027] Extracting the genome of Corynebacterium bacilli SYPA5-5 as a template;
[0028] PCR reaction conditions: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 30 s, annealing at 58°C for 30 s, extension at 72°C for 60 ...
Embodiment 2
[0030] Embodiment 2 Expression of mutant N-acetylglutamate kinase and Ni-NTA purification
[0031] Inoculate the recombinants stored in cryopreserved tubes into LB medium containing kanamycin (final concentration: 50 μg / mL), culture overnight at 37°C with shaking, transfer at 1% inoculum size, and culture at 37°C until the OD is about 0.6- 0.8, add human IPTG to a final concentration of 1 mmol / L, and induce expression overnight at 16°C. Centrifuge the overnight induced expression bacterial solution at 10000r / min, 4°C for 15min, collect the bacterial cells, suspend the bacterial cells with Tris-HCI (pH8.0) buffer, break the cells by ultrasonic, and then filter through a 0.45μm filter membrane, select the expression The vector pET-28a contains 6His-Tag, NAGK is purified by Ni-NTA, and the pure NAGK enzyme protein is analyzed by SDS-PAGE, and a specific band with a molecular weight of about 36kDa is detected. The pure NAGK enzyme is used for protein The concentration and enzyme ...
Embodiment 3
[0032] The catalytic constant comparison of embodiment 3 wild enzyme and mutant enzyme
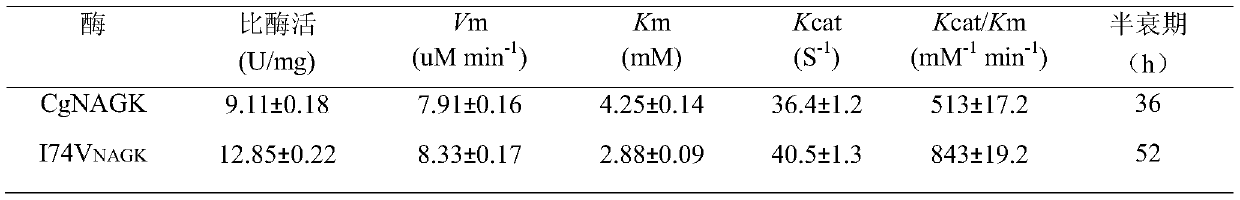
[0033] The pure enzyme solution obtained in the previous step was subjected to an enzymatic reaction: the total volume of the enzymatic reaction was 3mL, containing 595mmol / LTris-HCl (pH8.0), 20mmol / L N-acetylglutamic acid, 20mmol / L MgCl 2 , 20mmol / L ATP disodium salt, 149mmol / L NH 2 0H·HCl and an appropriate amount of crude enzyme solution; after reacting at 37°C for 1 h, add 1 mL of reaction termination solution (1.0mol / L HCI containing 5% FeCl3 ·6H 2 0,4% trichloroacetic acid) to stop the reaction; centrifuge, take the supernatant to measure the absorbance value A of N-acetylglutamic acid hydroxamic acid 540 . The values of Km, Vm and Kcat were measured by changing the concentration of the substrate N-acetylglutamic acid (NAG) and using the double reciprocal method. The results obtained are shown in Table 1: N-acetylglutamate kinase mutant I74V NAGK Catalytic efficiency (Kcat / Km) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com