Preparation method of Fe3O4@Au nuclear shell function material
A functional material, core-shell technology, applied in the field of nanomaterials with magnetic core-shell structure, can solve the problems of easy agglomeration of gold nanoparticles, easy agglomeration of particles, uneven size, etc., and achieves good dispersibility, uniform particle size distribution, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
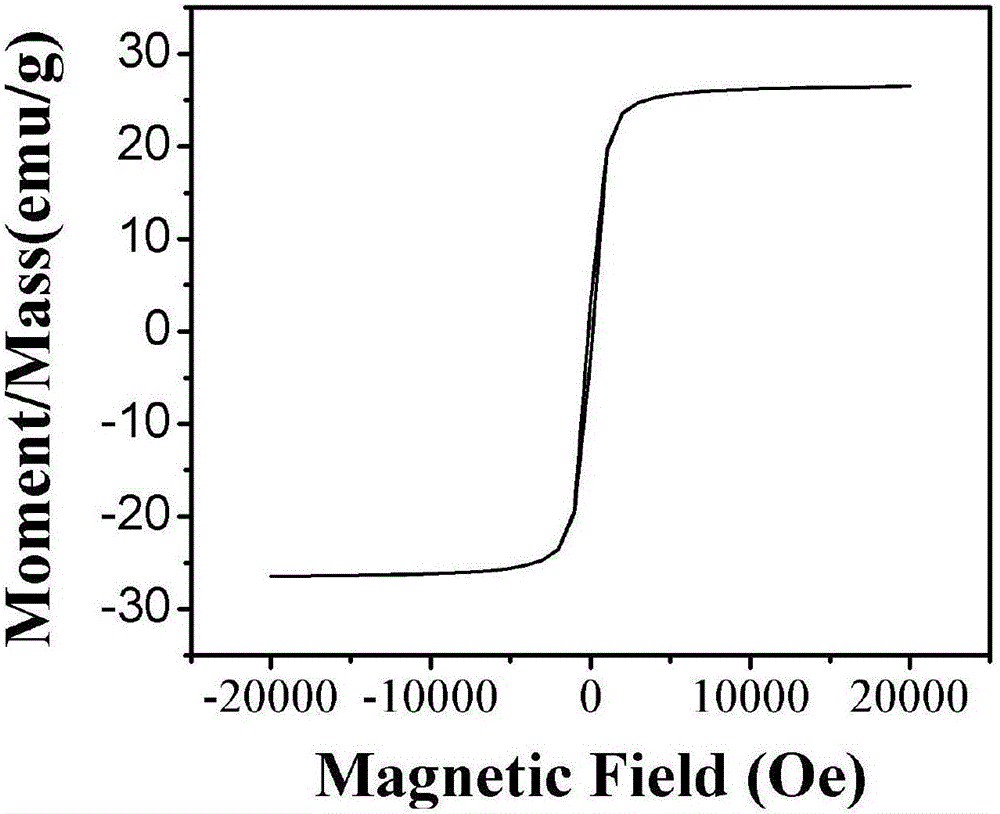
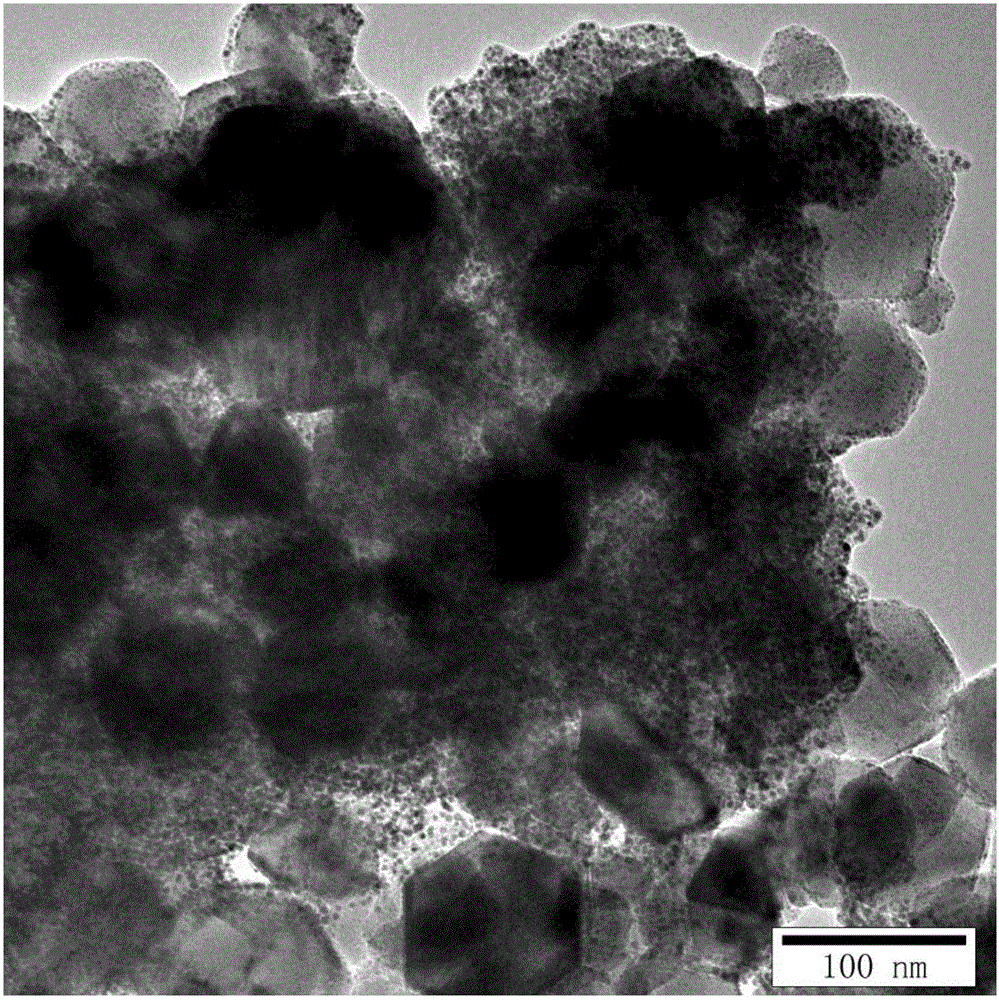
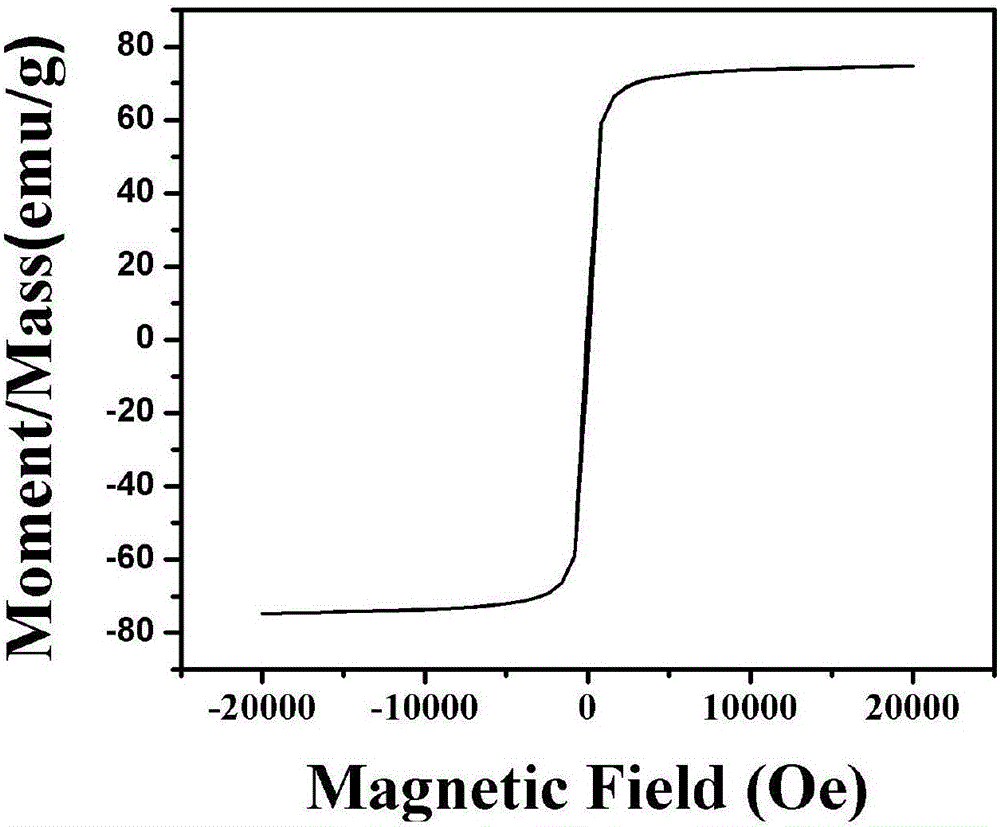
Image
Examples
Embodiment 1
[0044] a kind of Fe 3 o 4 The preparation method of @Au core-shell functional material comprises the following steps:
[0045] S1, the Fe 3 o 4 The nanoparticles were added into methanol and mixed to obtain solution A;
[0046] S2. Take polyethyleneimine and methanol for shaking and dissolving, add potassium hydroxide and carbon disulfide, stir for 10 minutes, add the solution A obtained in S1 dropwise, shake for 5 minutes, let stand, take the precipitate and wash it with water to obtain Fe 3 o 4 @PEI-DTC nanoparticles; the Fe 3 o 4 Add @PEI-DTC nanoparticles into water and mix to obtain solution B;
[0047] S3. Add the solution B obtained in S2 dropwise to the chloroauric acid aqueous solution, shake continuously during the dropwise addition, then let it stand, take the precipitate and wash it with water, add water and mix it to obtain solution C; add hydroboration dropwise to solution C Sodium aqueous solution, continuously shake during the dropping process, then sta...
Embodiment 2
[0049] a kind of Fe 3 o 4 The preparation method of @Au core-shell functional material comprises the following steps:
[0050] S1. Using iron acetylacetonate, oleic acid and benzyl ether as raw materials, in an argon atmosphere, heating to reflux for redox reaction to obtain Fe 3 o 4 Nanoparticles; the Fe 3 o 4 After the nanoparticles are washed with methanol, they are added to methanol and mixed to obtain solution A;
[0051] S2. Take polyethyleneimine and methanol for shaking and dissolving, add potassium hydroxide and carbon disulfide, stir for 13 minutes, add the solution A obtained in S1 dropwise, shake for 6.5 minutes, let it stand, take the precipitate and wash it with water to obtain Fe 3 o 4 @PEI-DTC nanoparticles; the Fe 3 o 4 Add @PEI-DTC nanoparticles into water and mix to obtain solution B;
[0052] S3. Add the solution B obtained in S2 dropwise to the chloroauric acid aqueous solution, shake continuously during the dropwise addition, then let it stand, t...
Embodiment 3
[0054] a kind of Fe 3 o 4 The preparation method of @Au core-shell functional material comprises the following steps:
[0055] S1. Using iron acetylacetonate, oleic acid and benzyl ether as raw materials, in an argon atmosphere, heat up to 290°C, reflux for 10 minutes, cool to room temperature, stand still to collect the precipitate, and use a mixed solvent composed of toluene and n-hexane in any proportion After cleaning, add in chloroform and store to obtain Fe 3 o 4 Nanoparticles; the Fe 3 o 4 After the nanoparticles were washed with methanol, they were added to methanol and mixed to obtain solution A, in which the volume ratio of oleic acid and benzyl ether was 1:11, and the weight-to-volume (g / ml) ratio of iron acetylacetonate and oleic acid was 0.65:1.6 , Fe 3 o 4 The weight-to-volume (mg / ml) ratio of nanoparticles and methanol is 1:8, among which, it is used to clean Fe 3 o 4 The amount of methanol used for nanoparticles is not included;
[0056] S2. Take poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com