Preparation method and application of nanopd1.6au1.0 alloy @mn(ii)mof
A pd1.6au1.0, MOF technology, applied in the preparation of imino compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc. The problems of less amines, etc., to achieve the effect of simple preparation method, easy recovery, and improved utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the synthesis of ligand L
[0050] in N 2 Under the protection of 4-bromoacetophenone (2.00g, 10.0mmol), pyridine-4-boronic acid (1.48g, 12.0mmol), tetrakistriphenylphosphine palladium (0.34g, 0.30mmol), anhydrous phosphoric acid Potassium (4.14g, 30.0mmol), 40ml of ethanol and 20ml of water were placed in a three-necked flask (50ml), and stirred at a constant temperature of 80°C for 36h. After the reaction was completed, the liquid was separated while it was hot, and the upper organic layer was taken, dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain a yellow solid. Perform column chromatography (CH 2 Cl 2 :CH 3 OH=20:1), a white intermediate was obtained. in N 2 Under the protection of , the intermediate (1.00g, 5.0mmol), sodium amide (0.39g, 5mmol), ethyl trifluoroacetate (3.6ml, 25mmol) and anhydrous tetrahydrofuran (30ml) were placed in a 100ml three-necked flask at room temperature Stir ...
Embodiment 2
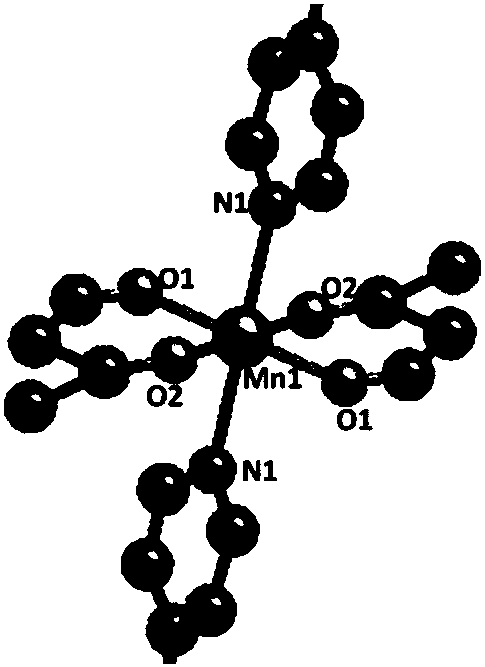
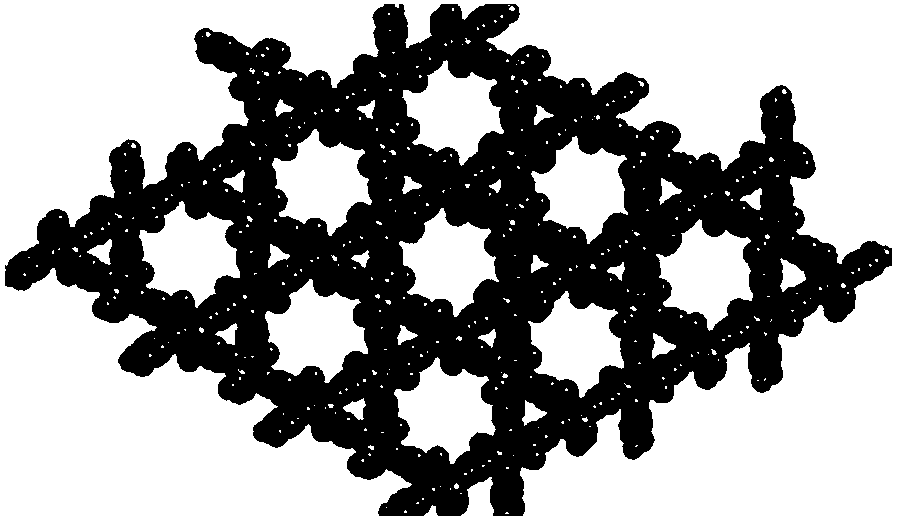
[0051] Embodiment 2: the synthesis of Mn (II)-MOF
[0052] Dissolve 3 mg of organic ligand L in 2 ml of ethyl acetate, 5 mg of manganese acetate in 2 ml of methanol, put it in a 10 ml test tube, add a mixture of ethyl acetate and methanol between the two solutions, and leave it at room temperature after 5 days Light red crystals were obtained with a yield of 82% (based on L).
[0053] We passed TGA, CO 2 The compound was characterized by adsorption and XRD, and the results are shown in Figure 5 , 7 ,9.
Embodiment 3
[0054] Embodiment 3: Nano Pd 1.6 Au 1.0 Synthesis of Alloy@Mn(II)-MOF
[0055] Weigh 5 mg of potassium tetrachloroalloy and 5 mg of palladium chloride and dissolve them in 5 ml of ethanol, and perform ultrasonic treatment to dissolve completely to obtain a dark brown solution. After weighing 5 mg MOFs and mixing with the above solution, it was placed on a magnetic stirrer and stirred for 1 h at a speed of 130 r / min. Then carry out centrifugation, firstly wash twice with ethanol, then wash twice with acetonitrile, and finally wash once with ether, and finally the solution is colorless, which proves that the washing is clean. The obtained product is baked under an infrared lamp for about 5 minutes to make it dry. Weigh 2mg of sodium borohydride and dissolve it in 2ml of secondary water, shake it slightly to dissolve completely, then slowly add the solution drop by drop to the dried product, a large number of bubbles will be observed, and the product will quickly turn black. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com