Recombinant buckwheat glutaredoxin as well as preparation method and application thereof
A protein and buckwheat technology, applied in the field of biotechnology and medical and health products, can solve the problems of protein expression, purification and functional analysis that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Screening and Analysis of Buckwheat Glutenedoxin Gene
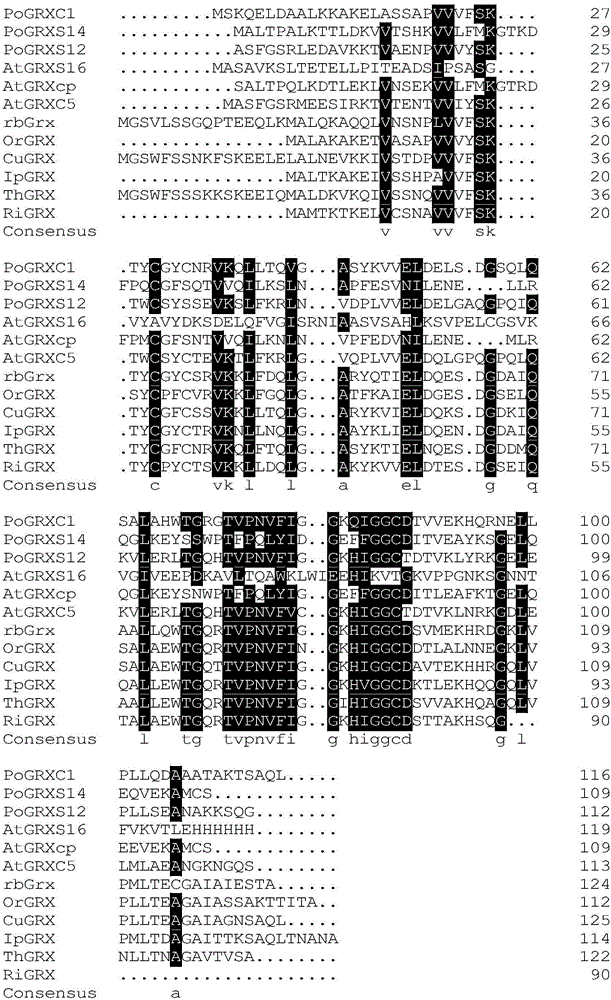
[0022] Buckwheat gluten redoxin (rbGrx) gene, the nucleotide sequence of which is SEQ ID NO:1. The gene was screened by analyzing the whole buckwheat gene sequence. Sequence alignment of rbGrx with Grx proteins from different plants (see figure 1 ), which included 12 Grx sequences from nine different plants. Among them, PoGRXC1 is from the National Institutes of Health database 2E7P_A, PoGRXS14 is from the National Institutes of Health database Accession:2LKU_A, PoGRXS12 is from the National Institutes of Health database Accession:3FZ9_A, AtGRXS16 is from the National Institutes of Health database Accession:2LWF_A, AtGRXcp is from the National Institutes of Health database Database Accession: 3IPZ_A, AtGRXC5 from the National Institutes of Health database Accession: 3RHB_A, OrGRX (Oryza sativa) from the National Institutes of Health database Accession: CAA54397, CuGRX (Cucumis sativus) from the National...
Embodiment 2
[0024] Example 2 Expression and purification of recombinant buckwheat glutaredoxin gene in Escherichia coli.
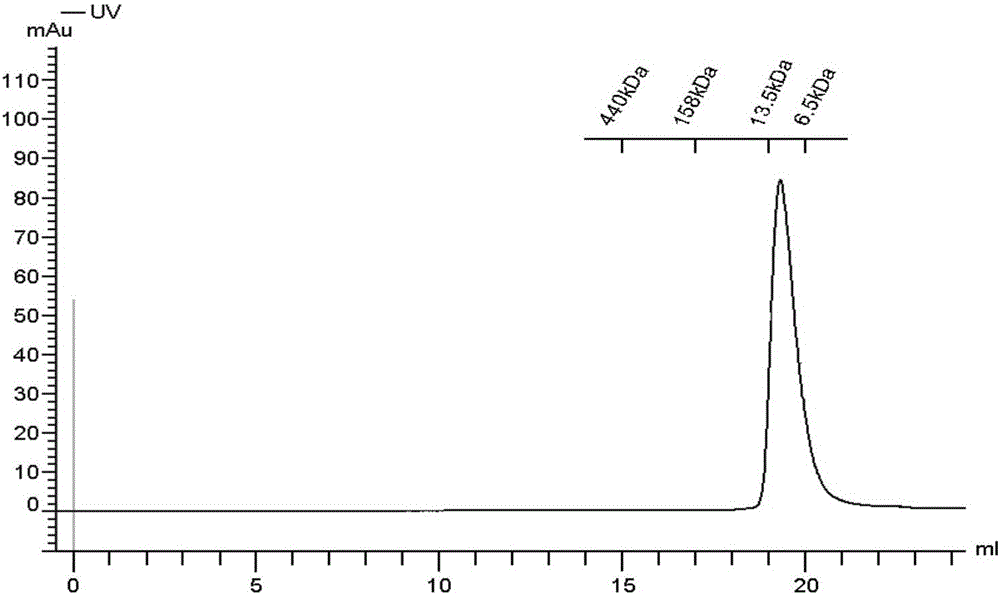
[0025] The rbGrx gene was constructed on the pGEX-6p-1 vector by genetic engineering technology. Transform the fusion protein expression plasmid constructed by cloning into BL21(DE3), inoculate it in 5 mL of LB culture overnight at 37°C, and transfer it to 800 mL of LB medium for expansion after 12 hours, and shake it at 37°C Cultivate in the bottle until the OD is about 0.4-0.6, reduce the culture temperature to 16°C after 4 hours, and then add IPTG (isopropyl-β-D thiogalactopyranoside) with a final concentration of 0.5mM to induce expression, about 16 - Collect bacteria by centrifugation after 20 hours. Use 50mM HEPES buffer (pH 7.8, containing 200mM NaCl) to suspend the cells, use a low-temperature ultra-high pressure cell disruptor to lyse the cells, separate the insoluble precipitate by centrifugation, and pass the supernatant obtained after high-speed centrifug...
Embodiment 3
[0026] Example 3 Determination of rbGrx enzyme activity of recombinant buckwheat gluteledoxin
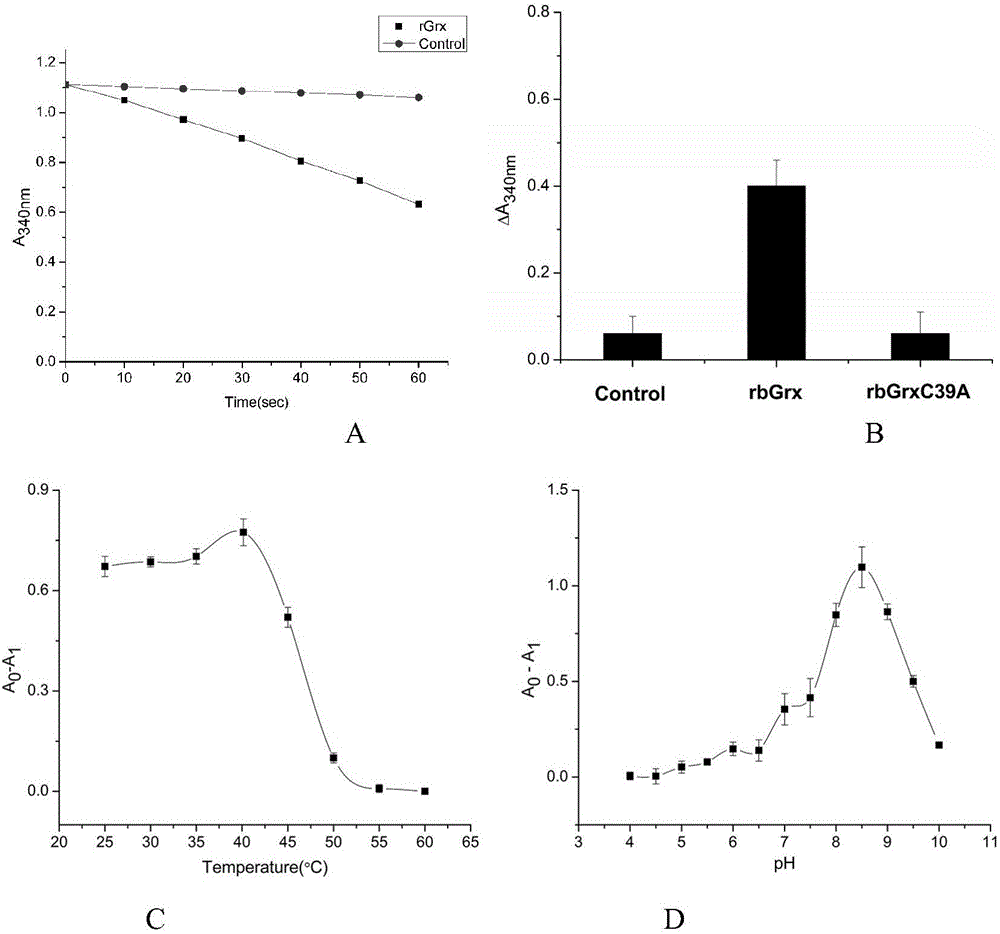
[0027]Determination of Grx activity of protein: the reaction system is 500 μL, which contains HEPEs (50mmol / L, pH 7.0), EDTA (1mmol / L), GSH (1mmol / L), NADPH (0.25mmol / L), 1U GR (2μmol / L L). Mix the reactants for pre-incubation for 3min, then add a final concentration of 0.5mmol / L HED (bisdihydroxyethyl disulfide) to start the reaction, and monitor the 340nm (ε NADPH =6220M -1 cm -1 ) at the change of NADPH absorbance value. Grx activity is defined as the amount of enzyme required to consume 1 μmol / L NADPH per minute at 25°C, that is, one activity unit. The specific activity of the rbGrx enzyme obtained through measuring is 300U / mg ± 15.0 (see image 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com