Solid electrolyte capable of lowering interface resistance on metal lithium electrode, and preparation method for solid electrolyte
A solid electrolyte and electrode interface technology, applied in the direction of electrolyte immobilization/gelation, circuits, electrical components, etc., can solve the problems of potential safety hazards, narrow applicable temperature range, and strong side reactions between electrodes and electrolytes, etc., to achieve Effects of reduced migration resistance, low equipment requirements, and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step 1: Weigh lithium carbonate, lanthanum oxide, and zirconium oxide according to the stoichiometric ratio, and grind them evenly by dry grinding method, so that the mixture is fully mixed, and then placed in an alumina crucible with a cover;
[0036] The second step: put the mixture in the first step in an alumina crucible with a cover, sinter in a muffle furnace at 900°C for 6 to 12 hours, and cool to room temperature at a heating and cooling rate of 3°C / min;
[0037] The third step: transfer the reactant in the third step to an agate mortar for grinding, mix well, and record it as mother powder A;
[0038]Step 4: Grind the reactant in the third step again in an agate mortar, mix thoroughly and evenly, and add 0.1% to 0.7% halogen lithium salt, which is recorded as mother powder B;
[0039] Step 5: Add 0.5g of the mother powders A and B in the third step and the fourth step into stainless steel molds, and press them in a 20MPa press to obtain tablets C and D with a d...
Embodiment 2
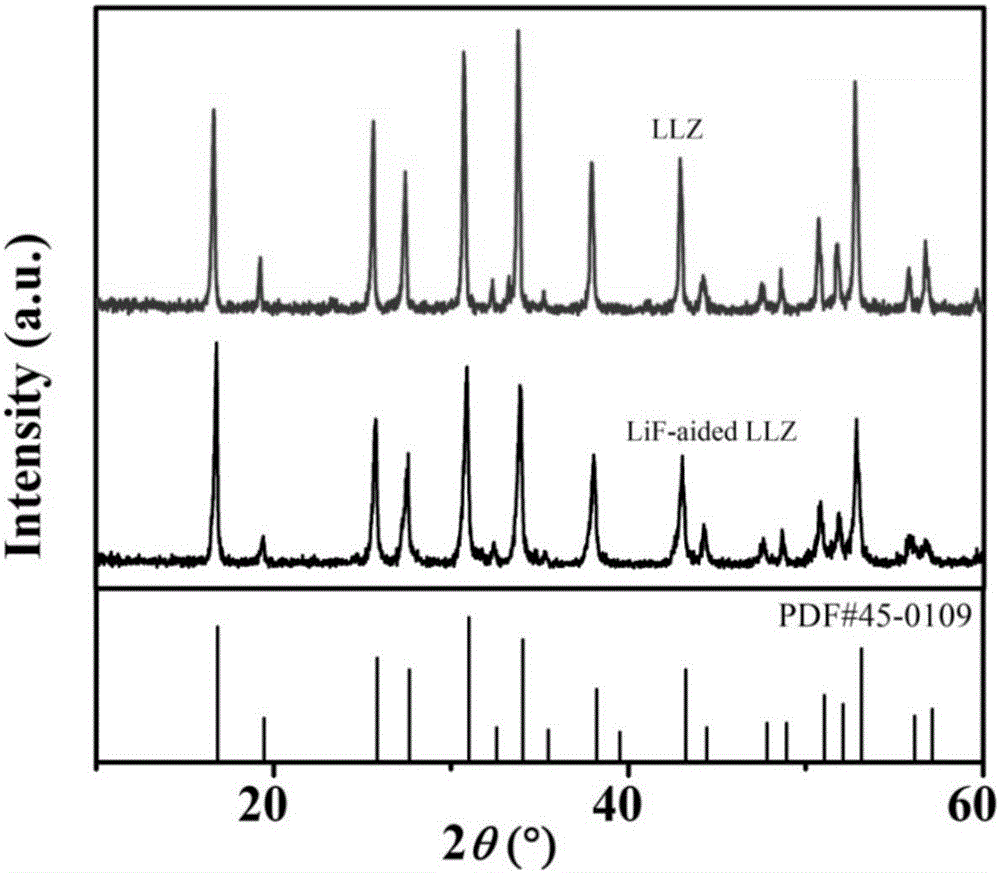
[0045] The preparation method in the present embodiment is identical with embodiment 1, by figure 1 It can be seen that the lithium-containing garnet XRD prepared in Example 1 is completely consistent with the standard spectrum (JCPDS NO.45-0109), which is defined as high-purity cubic phase lithium-containing garnet; doped with halogen lithium salt Lithium-containing garnet has not changed its crystal structure, has good crystallinity and no impurities.
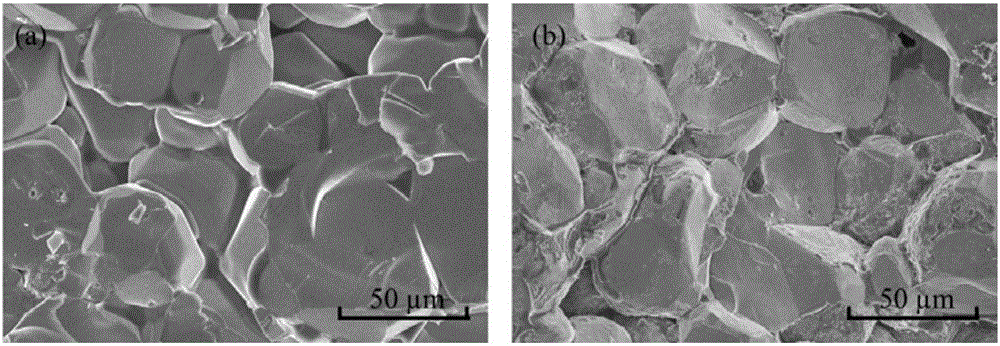
[0046] Depend on figure 2 It can be seen that lithium-containing garnet or lithium-halogen salt doped lithium-containing garnet has a dense structure, with a diameter of about 50-100 μm, no obvious pores, and a relative density of more than 90%, which proves that the dry grinding method combined with high-temperature solid-state sintering Non-porous lithium-containing garnet ceramic sheets were prepared by this method.
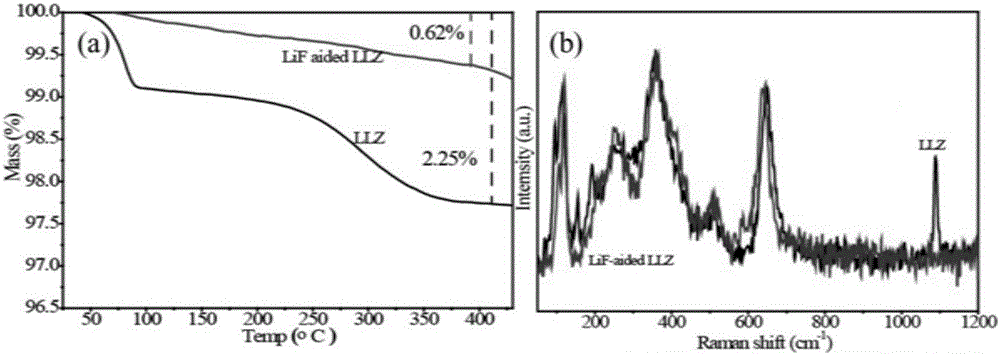
[0047] Utilize the lithium-containing garnet prepared in this embodiment 2 and the halogen lithium salt...
Embodiment 3
[0050] The preparation method in this example is the same as that in Example 1, the only difference is that the conditions in the preparation process are different, the pre-calcination reaction time in step 2 of this example is 6h, and the other conditions remain unchanged.
[0051] The phase of the lithium-containing garnet prepared in Example 3 was characterized by X-ray diffraction and other means. X-ray diffraction shows that the lithium-containing garnet obtained in Example 3 is a tetragonal phase, containing a small amount of cubic phase, and the reaction raw materials basically disappear without dense particles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com