Iron sucrose injection and preparation method thereof
A technology of iron sucrose and injection, applied in the field of medicine, can solve the problems such as the research on the bioequivalence of the unseen preparations, the complex process of repeated adsorption by activated carbon, and the reduction of the detection rate of visible foreign matter, and achieves the convenience of industrialized production operation, The effect of improved sterility assurance level and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] formula:
[0038]
[0039] Preparation steps:
[0040] 1) Add 80% of the total amount of water for injection into the preparation tank, adjust the water temperature to 15°C-30°C, add the prescribed amount of sodium gluconate and sucrose in sequence, and stir to dissolve;
[0041] 2) Add the benzyl alcohol of prescription quantity, stir and mix evenly;
[0042] 3) Continue to add the main active ingredient iron sucrose in the prescribed amount, and stir until completely dissolved;
[0043] 4) Slowly add 1M sodium hydroxide solution or 1M hydrochloric acid solution, adjust the pH of the solution to 10.5-11.1, and mix well;
[0044]5) Add water for injection to the full amount.
[0045] The injection obtained in step 5) is sterilized and filtered with two-stage 0.22um hydrophilic polyvinylidene fluoride filters, collected in a buffer tank, filled into glass ampoules with about 5ml / bottle, and sealed; The intermediate product is transferred to the product sterilizer,...
Embodiment 2
[0048] Thermodynamic stability evaluation of iron sucrose injection
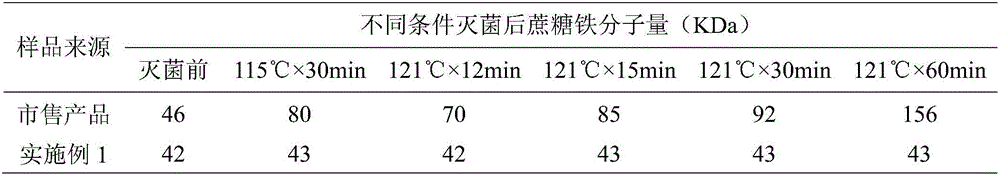
[0049] The filling intermediate product prepared in Example 1 (before sterilization) and appropriate amount of commercially available products were divided into 6 groups, wherein 1 group was not subjected to terminal moist heat sterilization, and the remaining 5 groups were respectively sterilized at 115°C×30min, 121°C×12min, 121°C. ℃×15min, 121℃×30min and 121℃×60min for moist heat sterilization, the above 6 groups of sample numbers are S2-1, S2-2, S2-3, S2-4, S2-5 and S2-6 respectively. After the sterilization, the molecular weight of iron sucrose in different samples was determined by referring to the method recorded in the monograph "Iron Sucrose Injection" of the United States Pharmacopoeia USP36-NF31.
[0050] The specific experimental results are shown in Table 1
[0051] Table 1. Detection results of iron sucrose molecular weight under different sterilization conditions
[0052]
[0053] The abov...
Embodiment 3
[0055] The prescription and process refer to the above example 1, the only difference is that the first type of stabilizer sodium gluconate in the prescription is adjusted to an equal amount of zinc gluconate or calcium gluconate to prepare two batches of samples; or, the prescription and process refer to The above Example 1 is only different in that the second type of stabilizer sucrose in the prescription is adjusted to use equal amounts of maltose and lactose to prepare two batches of samples.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com