Method for preparing triphosadenine by enzymic method
An enzymatic preparation technology of adenosine triphosphate, which is applied in the field of enzymatic preparation of adenosine triphosphate, can solve problems such as unclear classification, and achieve the effects of easy control, simple production process, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
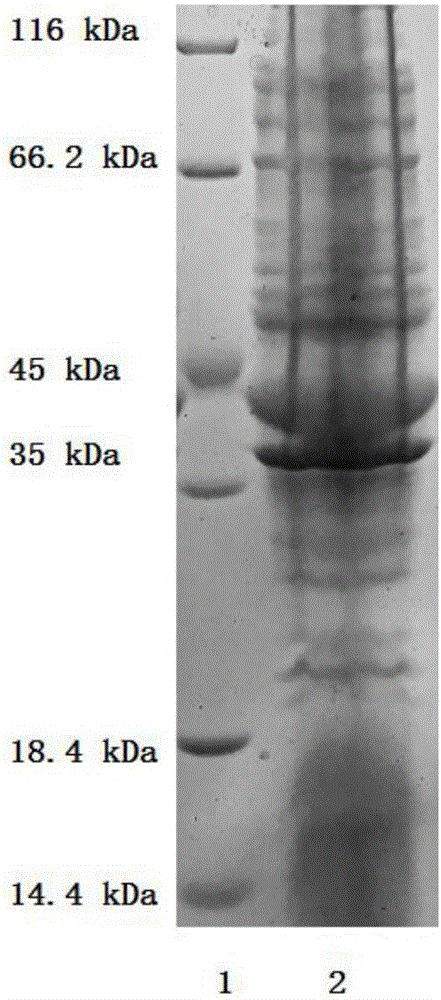
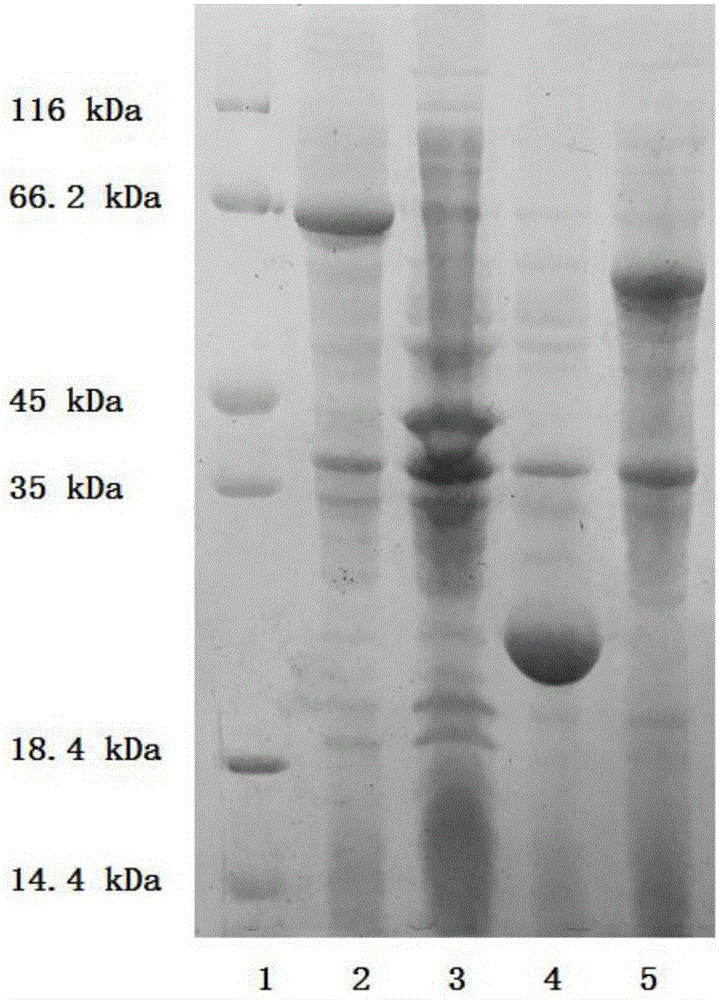
[0046] The preparation of embodiment 1 AK, Ppk, Adk and Pap enzyme
[0047] The AK, Ppk (Ppk1 and / or Ppk2), Adk and Pap enzymes in the method of the present invention can be obtained commercially, or are enzymes with the same catalytic function after artificial modification.
[0048] The preparation process of AK, Ppk1, Ppk2, Adk and Pap enzymes is as follows:
[0049] According to the sequences of the five enzyme genes, five pairs of amplification primers were designed. Extract Saccharomyces cerevisiae (Saccharomycescerevisiae) bacterial strain genomic DNA, use it as a template, PCR amplify ado1 (AK enzyme gene) fragment, and connect it to pET22b carrier (purchased from Novagene Company); Extract Escherichia coli (Escherichia coli) K12 bacterial strain ( Purchased from Tiangen Biochemical Technology Co., Ltd.) genomic DNA, using it as a template, PCR amplified ppk1 and adk gene fragments, and connected them to the pET 22b vector (purchased from Novagene); extract Pseudomonas...
Embodiment 2
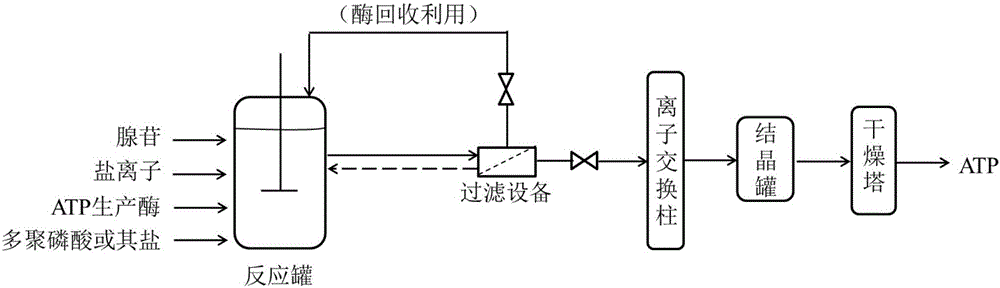
[0057] Embodiment 2 uses free enzyme to prepare ATP
[0058] image 3 Process flow diagram for the production of ATP using free enzymes in the method of the present invention. see image 3 , the operation steps of using free enzyme to prepare ATP are as follows:
[0059] (1) The reaction of synthesizing ATP in the reaction tank:
[0060] In the reaction tank, the reaction system of 100L sterile water contains substrate 2.5kg adenosine, and 0.1kg ATP, 0.5kg sodium dihydrogen phosphate, 0.3kg sodium chloride, 0.3kg ammonium sulfate, 1.0kg magnesium chloride hexahydrate and 2.0kg sodium hexametaphosphate solution, stir evenly during preparation to prevent precipitation. The pH value was adjusted to 7.5, and 500U / L Pap enzyme, 800U / LPpk2 enzyme and 800U / L Pap enzyme were added to the reaction system to start the reaction. During the reaction, the pH value was controlled to be 7.5, and the temperature was 45°C.
[0061] Figure 5 , Figure 6 Respectively, the HPLC detection...
Embodiment 3
[0068] Embodiment 3 uses free enzyme to prepare ATP
[0069] see image 3 , the operation steps of using free enzyme to prepare ATP are as follows:
[0070] (1) The reaction of synthesizing ATP in the reaction tank:
[0071] In the reaction tank, the reaction system of 100L sterile water contains substrate 3.0kg adenosine, and 0.1kg AMP, 0.5kg disodium hydrogen phosphate, 0.3kg potassium chloride, 0.25kg ammonium chloride, 2.5kg heptahydrate sulfuric acid The solution of magnesium and 3.0kg tetrapolyphosphoric acid should be uniformly stirred during preparation to prevent precipitation. Adjust the pH value to 7.0, add 500U / LAK enzyme, 500U / LPpk2 enzyme, 300U / LAdk enzyme and 500U / L Pap enzyme to the reaction system to start the reaction. During the reaction, the pH value was controlled to be 7.0, and the temperature was 40°C.
[0072] After 8 hours of reaction, the amount of ATP produced was about 50 g / L, and the conversion rate of adenosine reached 85%. The HPLC detection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com