A kind of neurotrophin sustained-release coacervate for curing the peripheral nerve injury and its application thereof
A technology for neurotrophic factors and peripheral nerve injury, which is applied to nervous system diseases, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc., to achieve wide and cheap raw material sources, high encapsulation rate, and remarkable therapeutic effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Establishment of an animal model of peripheral nerve injury
[0029] a. Rat sciatic nerve crush injury model
[0030] SPF grade Wistar rats were selected and raised in separate cages in the school experimental animal center, kept at room temperature of 22°C, with natural light, and free to eat. Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate successively, and the sciatic nerve of the right leg was incised, and the sciatic nerve was separated with a glass minute needle. For severe contusions, the muscles and skin were sutured with non-invasive fine thread (5 / 0) and disinfected with povidone iodine. The sham operation group only needs to incision and suture. The temperature was maintained at 37°C throughout the operation to prevent the death of the modeled Wistar rats due to hypothermia.
[0031] b. Rat sciatic nerve transection model
[0032]SPF grade Wistar rats were selected and raised in separate cages in the school experiment...
Embodiment 2
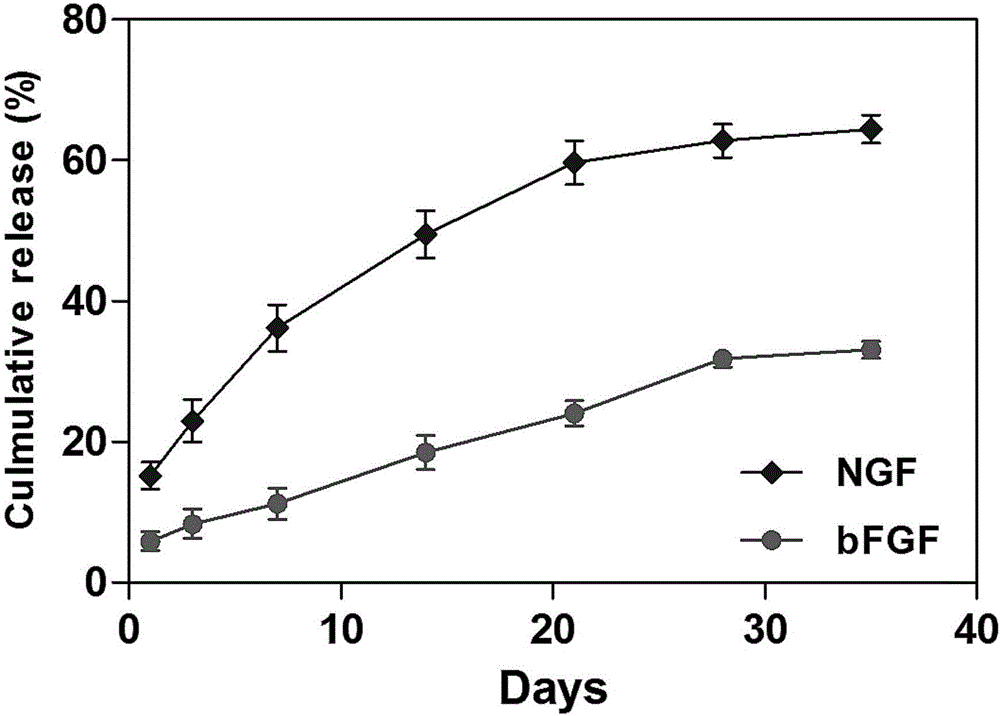
[0033] Example 2. Preparation of slow-release aggregates of neurotrophic factors
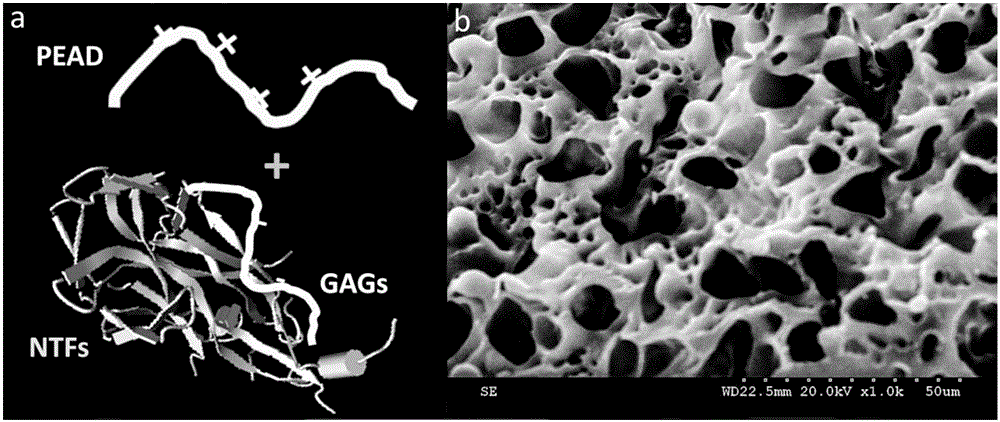
[0034] a. Synthesis of PEAD:
[0035] First, 1000 mg of ethylene glycol diglycidyl ether (EGDE), 1340 mg of aspartic acid (ASP) protected by tert-butoxycarbonyl (t-BOC) and 50 mg of tertiary ammonium bromide (TBAB) were put into 10 mL After adding 0.6mL of dimethylformamide (DMF) to the microwave reaction flask and mixing it well, heat it in a microwave reactor at 120°C for 20min to obtain a viscous orange liquid; then add 5mL of trifluoro Acetic acid (TFA) and 20 mL of dichloromethane (DCM) were stirred at room temperature for 2 h to extract the intermediate polyethylene-aspartic acid glyceride (PED); then 770 mg of ASP, 323 mg of N-hydrosuccinimide (NHS), 753 mg of di Cyclohexylcarbodiimide (DCC) was added to 35mL of DMF solution and stirred in the glove box for 2h to obtain a milky orange liquid with precipitation, which was sucked into a 50mL centrifuge tube and placed in a high-speed centr...
Embodiment 3
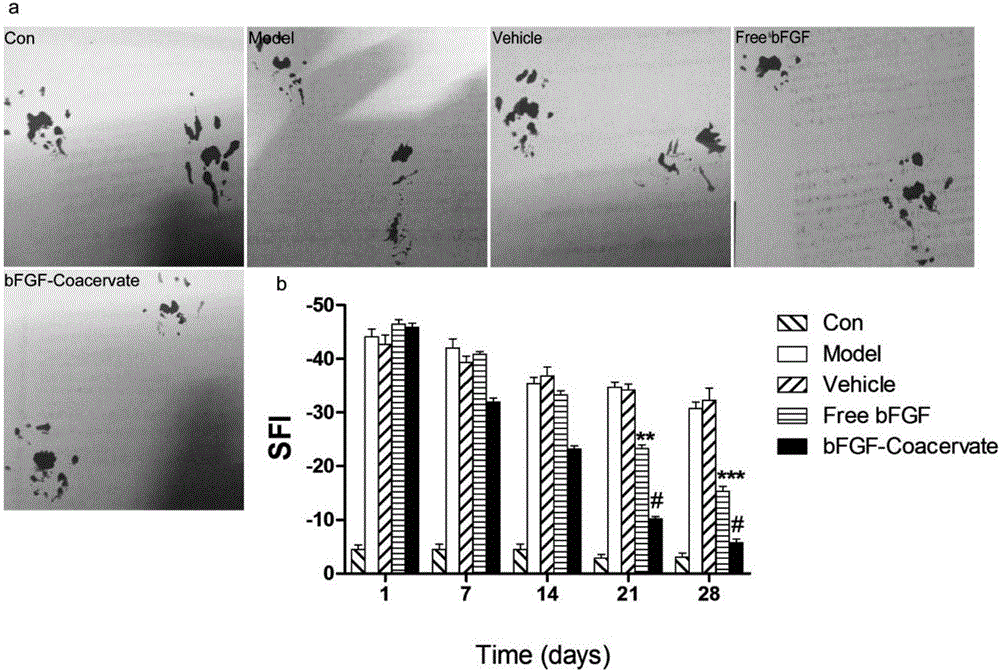
[0047] Embodiment 3. animal administration and curative effect evaluation
[0048] After suturing the muscles and skin of Wistar rats with sciatic nerve crush injury or transverse injury model, the slow-release aggregates of neurotrophic factors prepared by each experimental group and control group in Table 1 were delivered into the injured sciatic nerve area in time through a 30G needle .
[0049] The evaluation indicators for the effect of nerve restoration are as follows:
[0050] a. Behavior: Wistar rats after sciatic nerve injury were tested for motor function and sensory function by footstep imprinting and hot plate test respectively, and the time was 1st, 2nd, 3rd and 4th weeks after administration;
[0051] b. Histomorphology: After 1 month of drug treatment, the rats in each group were killed, the injured sciatic nerve was collected, pathological sections were made, and HE staining, Massion staining and transmission electron microscopy were performed;
[0052] c. Ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com