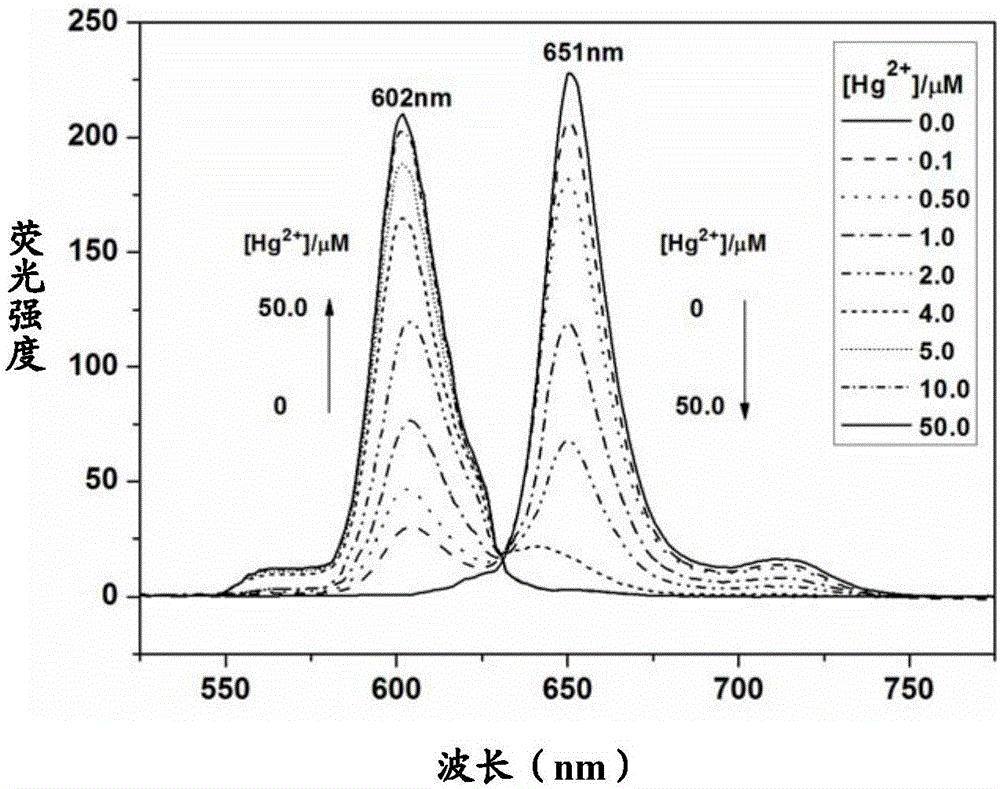
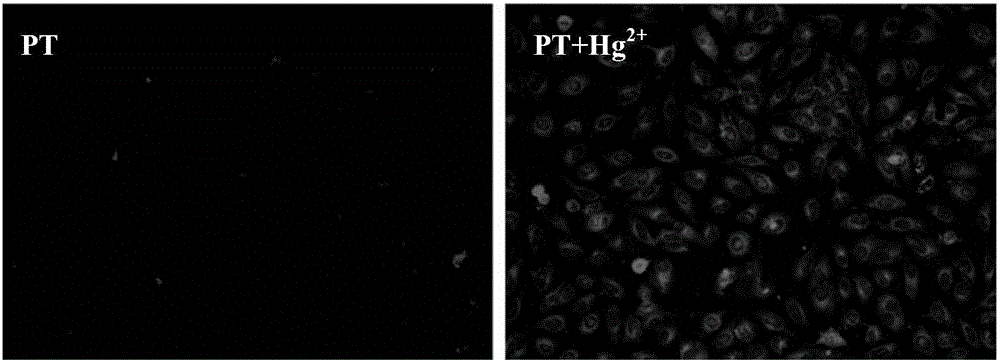
Preparing and application of a reactive mercury ion fluorescence probe
A fluorescent probe and mercury ion technology, applied in fluorescence/phosphorescence, analysis through chemical reaction of materials, luminescent materials, etc., can solve problems such as background interference, complicated preparation, poor water solubility, etc. The effect of simple processing and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Tetraphenylporphyrin (TPP) (800 mg, 1.31 mmol) was dissolved in trifluoroacetic acid (50 mL), and sodium sulfite (100 mg, 1.45 mmol) was added. After reacting at room temperature for 3 min, 500 mL of water was added to quench the reaction. The reaction solution was extracted with dichloromethane, and the organic layer was washed with saturated aqueous sodium bicarbonate solution and distilled water, respectively, and rotary evaporated to obtain a purple solid. Silica gel column separation, dichloromethane as the developing solvent, to collect the second color band product. The solvent was evaporated to dryness by rotary evaporation, and concentrated hydrochloric acid (50 mL) and stannous chloride (1100 mg, 4.875 mmol) were continuously added. The mixture was heated to 65° C., and reacted for 1 h under the protection of argon, and then 500 mL of distilled water was added. Add ammonia water to neutralize to pH 8. The reaction liquid was extracted with dichloromethane, ...
Embodiment 2
[0056] Tetraphenylporphyrin (TPP) (800 mg, 1.31 mmol) was dissolved in trifluoroacetic acid (50 mL), and sodium sulfite (100 mg, 1.45 mmol) was added. After reacting at room temperature for 3 min, 500 mL of water was added to quench the reaction. The reaction solution was extracted with dichloromethane, and the organic layer was washed with saturated aqueous sodium bicarbonate solution and distilled water, respectively, and rotary evaporated to obtain a purple solid. Silica gel column separation, dichloromethane as the developing solvent, to collect the second color band product. The solvent was evaporated to dryness by rotary evaporation, and concentrated hydrochloric acid (50 mL) and stannous chloride (1100 mg, 4.875 mmol) were continuously added. The mixture was heated to 65° C., and reacted for 1 h under the protection of argon, and then 500 mL of distilled water was added. Add ammonia water to neutralize to pH 8. The reaction liquid was extracted with dichloromethane, ...
Embodiment 3
[0062]Tetraphenylporphyrin (TPP) (800 mg, 1.31 mmol) was dissolved in trifluoroacetic acid (50 mL), and sodium sulfite (100 mg, 1.45 mmol) was added. After reacting at room temperature for 3 min, 500 mL of water was added to quench the reaction. The reaction solution was extracted with dichloromethane, and the organic layer was washed with saturated aqueous sodium bicarbonate solution and distilled water, respectively, and rotary evaporated to obtain a purple solid. Silica gel column separation, dichloromethane as the developing solvent, to collect the second color band product. The solvent was evaporated to dryness by rotary evaporation, and concentrated hydrochloric acid (50 mL) and stannous chloride (1100 mg, 4.875 mmol) were continuously added. The mixture was heated to 65° C., and reacted for 1 h under the protection of argon, and then 500 mL of distilled water was added. Add ammonia water to neutralize to pH 8. The reaction liquid was extracted with dichloromethane, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com