H7N9 subtype avian influenza genetic engineering vaccine taking baculovirus as carrier as well as preparation method and application of vaccine
A genetically engineered vaccine and baculovirus technology, applied in the field of H7N9 subtype avian influenza genetically engineered vaccine and its preparation, can solve the problems of chicken embryo production limitation, hidden biological safety hazards, antigen pollution, etc., and achieve the convenience of large-scale production, Facilitate mass production and improve the expression level effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
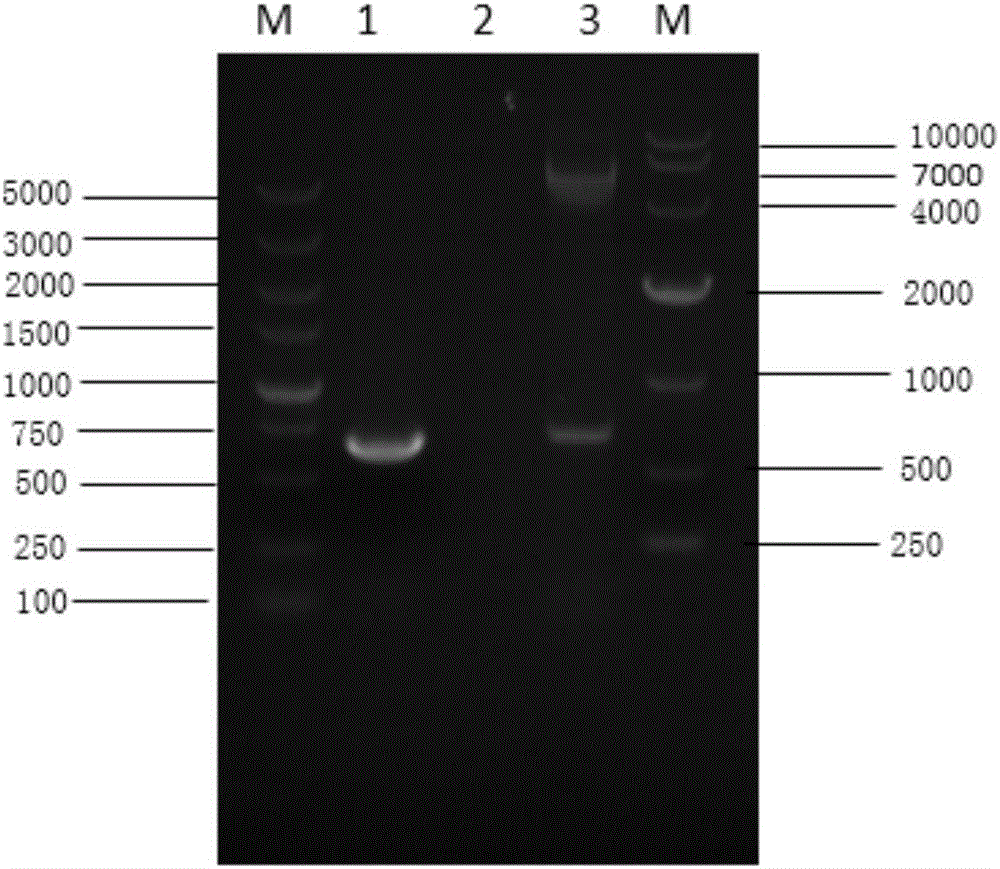
[0028] Embodiment 1: pFast-(CMV) in the present invention 2 Construction of Backbone Plasmids
[0029] (1) Construction and identification of recombinant plasmid pFast-CMV
[0030] Using the pcDNA3.1(+) plasmid as a template, two pairs of primers were designed, P1, P2 and P3, P4. Among them, primers P1 and P2 were used to amplify the human cytomegalovirus CMV promoter, and BstZ 17I and BbsI restriction sites were introduced upstream and downstream, and the primer sequences were as follows:
[0031] P1: 5'-GTATACGTTGACATTGATTATTGACTAGTTATTAATAGTAATCAATT-3'
[0032] P2: 5'-GAAGACTTGATCACACAGCTTGGGTCTCCCTATAGTGAGT-3'
[0033] The amplification conditions of primers P1 / P2 were: pre-denatured at 94°C for 5 minutes and then cycled; cycle parameters were: 94°C, 45s, 56°C, 45s, 72°C, 1min; after 30 cycles, final extension at 72°C for 10 minutes. The PCR product was recovered and digested with BstZ 17I and BbsI, and then inserted into the vector pFastBac TM Dual's BstZ 17I and Bbs...
Embodiment 2
[0038] Embodiment 2: Construction and identification of recombinant bacmid (Bacmid)
[0039] (1) Extraction of H7N9 subtype avian influenza virus genome RNA:
[0040] The H7 subtype avian influenza virus A / CK / GD / G1 / 2013 (H7N9) (hereinafter referred to as G1) used in this experiment and the positive serum prepared from it were all provided by the National Center for Prevention and Control of Zoonotic Diseases of South China Agricultural University. Saved by Joint Engineering Laboratories. The chicken embryo allantoic fluid containing G1 virus was centrifuged at 12,000 r / min for 5 min, and 500 μL of the supernatant was taken into a DEPC-treated Eppendorf tube to extract RNA.
[0041] ① Add 500 μL Trizol LS Reagent, shake vigorously and place at room temperature for 10 minutes, add 200 μL chloroform, mix thoroughly, wait for the emulsified solution to become milky, leave it at room temperature for 10 minutes, and then centrifuge (13000rpm, 15min, 4°C);
[0042] ② Transfer 600 μ...
Embodiment 3
[0059] Embodiment 3: recombinant baculovirus BV-(CHA) 2 acquisition and identification of
[0060] (1) Recombinant baculovirus BV-(CHA) 2 the acquisition
[0061] Using liposome-mediated transfection, the recombinant bacmid-(CHA) 2 Transfect sf9 insect cells and culture at 27°C. When the cells were cultured for 72 hours, lesions appeared in the cells, and the cell culture supernatant was collected to obtain the first-generation recombinant baculovirus (P1)BV-(CHA) 2 .
[0062] (2) Recombinant baculovirus BV-(CHA) 2 Transduction of PK15 cells and identification of its expression ability
[0063] 1 day before transduction, the well-formed PK15 cells were divided into 1 × 10 5 The amount of each well was inserted into a 6-well cell culture plate, and cultured in a 37°C incubator. After the cells grew to 70%-80% monolayer, the medium was removed, and the cells were washed with PBS containing calcium and magnesium ions. After mixing the recombinant baculovirus and PBS at a ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com