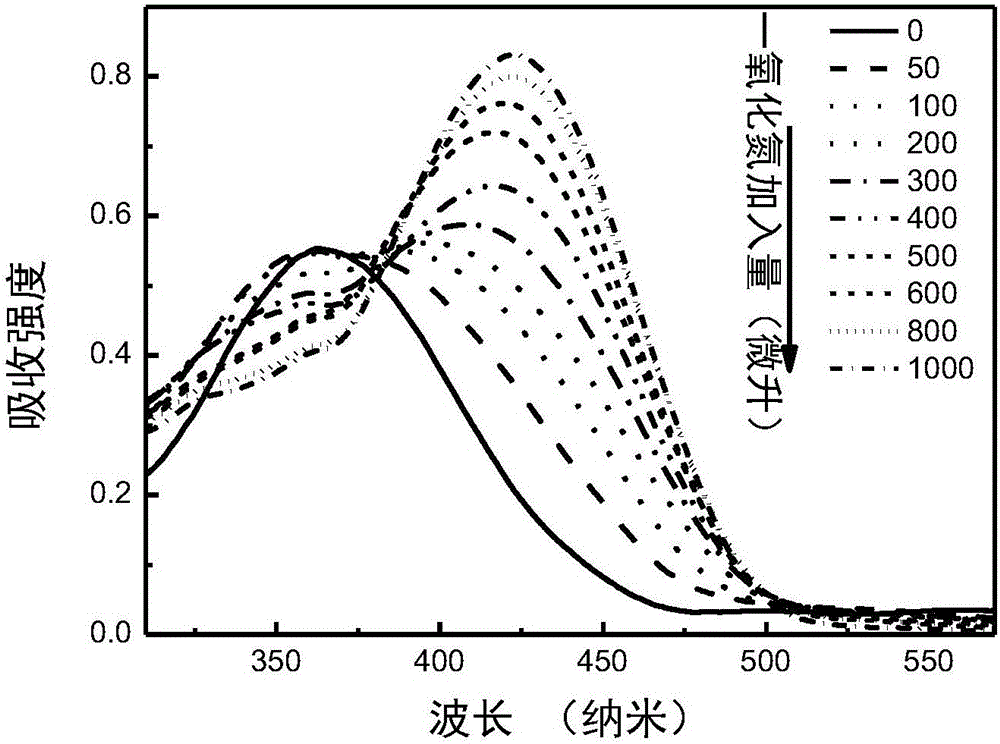
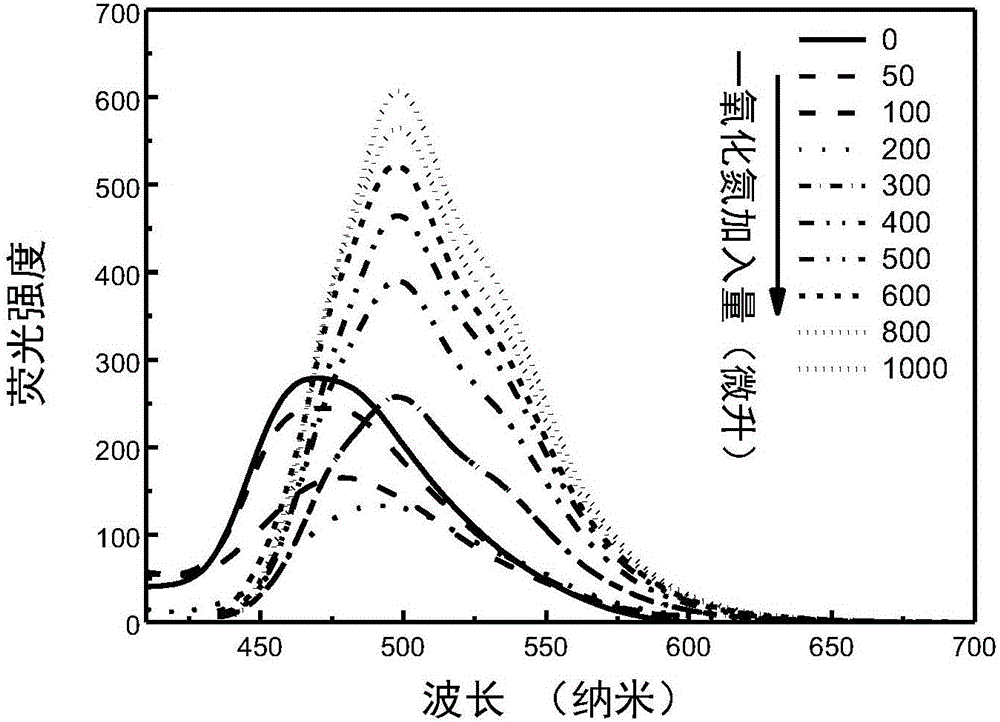
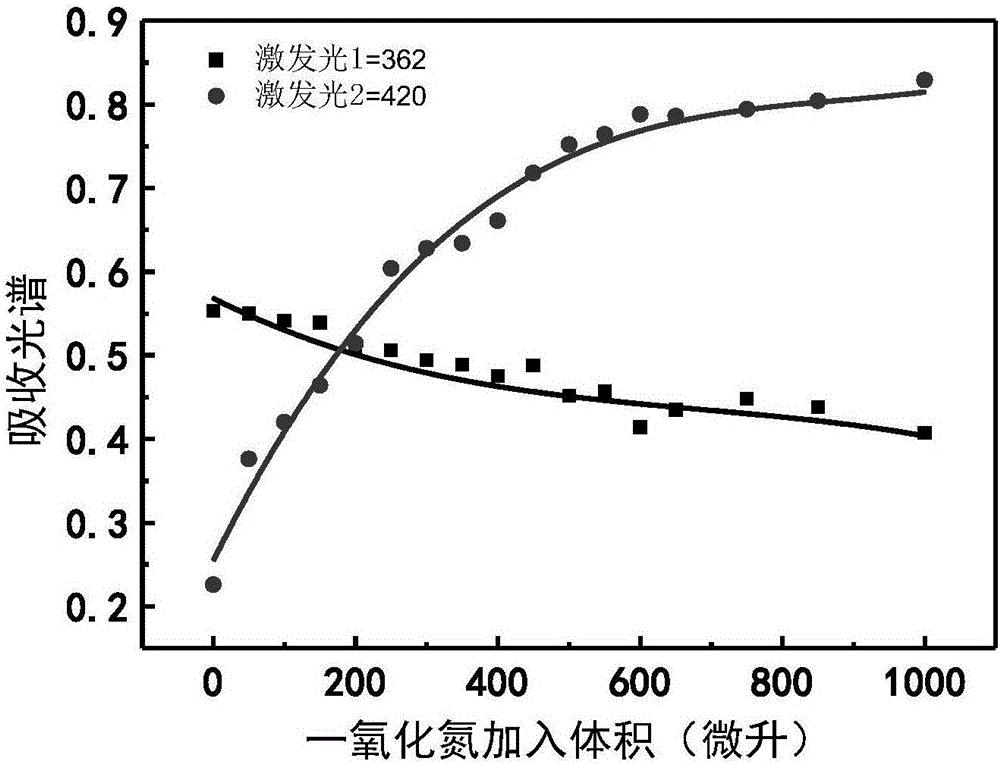
Nitric oxide probe based on fluorescent double-response mechanism and synthesis and application thereof
A nitric oxide and fluorescent probe technology, applied in the field of organic photochemistry, can solve the problems of background fluorescence interference, low detection sensitivity, semi-quantitative and quantitative detection of few cells, and achieve short response time, high sensitivity and good application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The molecular structure of the probe molecular material A-1 prepared in this example is:
[0053]
[0054] The present embodiment 1 is prepared by the preparation method (1), and the specific steps are:
[0055] i) In a two-neck flask (100mL), dissolve 4,7-dibromo-2,1,3-benzothiadiazole (2.01g, 6.84mmol), 2-thiopheneboronic acid (1.42g, 11mmol) in 20mL toluene 15 mL of 2M potassium carbonate solution was injected into the mixture, and the gas was exchanged three times. Under nitrogen protection, tetrakis(triphenylphosphine)palladium (150 mg, 0.14 mmol) was added, heated to 75° C., and reacted for 2-8 hours. Naturally cooled to room temperature, extracted with dichloromethane, dried, concentrated, and separated by column chromatography to obtain 1.73 g of red crystal 4,7-dithiophene-2,1,3-benzothiadiazole with a yield of 84.3%. 1 H NMR (400MHz, CDCl 3 )δ8.12(dd, J=3.7,1.1Hz,2H),7.88(s,2H),7.46(dd,J=5.1,1.1Hz,2H),7.24–7.20(m,2H). 13 C NMR (101MHz, CDCl 3 ) δ152.61,...
Embodiment 2
[0060] The molecular structure of the probe molecular material B-1 prepared in this example is:
[0061]
[0062] The present embodiment 2 is prepared by the preparation method (1), and the specific steps are:
[0063] i) The preparation process of 4,7-dithiophene-2,1,3-benzothiadiazole is the same as step i) of Example 1.
[0064] ii) In a two-necked flask (250ml), 1-bromo-2-(2-(2-methoxyethoxy)ethoxy)ethane (7.3g, 27.4mmol), dibromofluorene (2.9g , 12mmol), potassium iodide (200mg, 1.2mmol) was dissolved in DMSO (100ml) and 50% (w / w) NaOH aqueous solution (30ml). The reaction mixture was at 60. Inversely stirred for 6h. Extracted with dichloromethane, dried, concentrated, and separated by column chromatography to obtain light yellow liquid 9,9-bis(3,3'-2-(2-(2-methoxyethoxy)ethoxy)ethane) - 4.9 g of 2-bromofluorene, yield 79.1%. 1 H NMR (400MHz, CDCl 3)δ7.65(dd, J=5.6,3.0Hz,1H),7.56–7.52(m,2H),7.46(dd,J=8.0,1.8Hz,1H),7.38(d,J=2.6Hz,1H ), 7.33(dd, J=6.2, 2.8Hz, 2H),...
Embodiment 3
[0068] The molecular structure of the probe molecular material C-1 prepared in this example is:
[0069]
[0070] The present embodiment 3 is prepared by the preparation method (2), and the specific steps are:
[0071] i) In a two-necked bottle (100mL), 4,7-dibromo-2,1,3-benzothiadiazole (2.01g-3.05, 6.84-10mmol), trimethylsilylacetylene (0.67g-1.0g , 15-22.5mmol) was dissolved in 20-40mL tetrahydrofuran, and 15-30mL triethylamine was injected. The gas was exchanged three times, and under the protection of nitrogen, tetrakis(triphenylphosphine)palladium (50-150 mg, 0.05-0.14 mmol) was added, and the mixture was reacted at 50-80° C. for 4-24 h. Naturally cooled to room temperature, extracted with dichloromethane, dried, concentrated, and separated by column chromatography to obtain yellow-green crystal 4,7-ditrimethylsilylethynyl-2,1,3-benzothiadiazole, yield 77- 84.3%. 1 H NMR (400MHz, CDCl 3 )δ8.22(dd,J=3.7,1.1Hz,2H),0.14(m,18H).
[0072] ii) In a two-neck flask (150m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com